Menu (linked Index)
Energy Types and Conversion Efficiencies
Last Update: December 3, 2025
- Introduction
- The 2 Fundamental Energy States (KE and PE)
- Types and Forms of Energy
- Energy Sources And Conversion Routes to Electricity Generation
- Energy Conversion Efficiencies for Various Technologies
- Appendix 1 – Energy Type and PE and/or KE Categorization
- Appendix 2 – Energy Type and PE and/or KE Categorization (Tables)
Introduction
This article
- defines the fundamental energy states: kinetic energy (KE) and potential energy (PE),
- categorizes the various types of energies that fall under these two categories,
- describes the various energy routes to electricity,
- and provides the output/input efficiencies for several key electric generating technologies.
Detailed energy categorizations are listed and tabulated in the appendices for quick reference.
The 2 Fundamental States of Energy
The two fundamental categories of energy are kinetic energy and potential energy.
Kinetic Energy
Kinetic energy is the energy of motion.
Any object that is moving, whether it’s a speeding car, a rolling ball, or the thermal movement of atoms, possesses kinetic energy, which depends on its mass and speed.
Picture: Kinetic Energy of Translation, Rotation, and Vibration
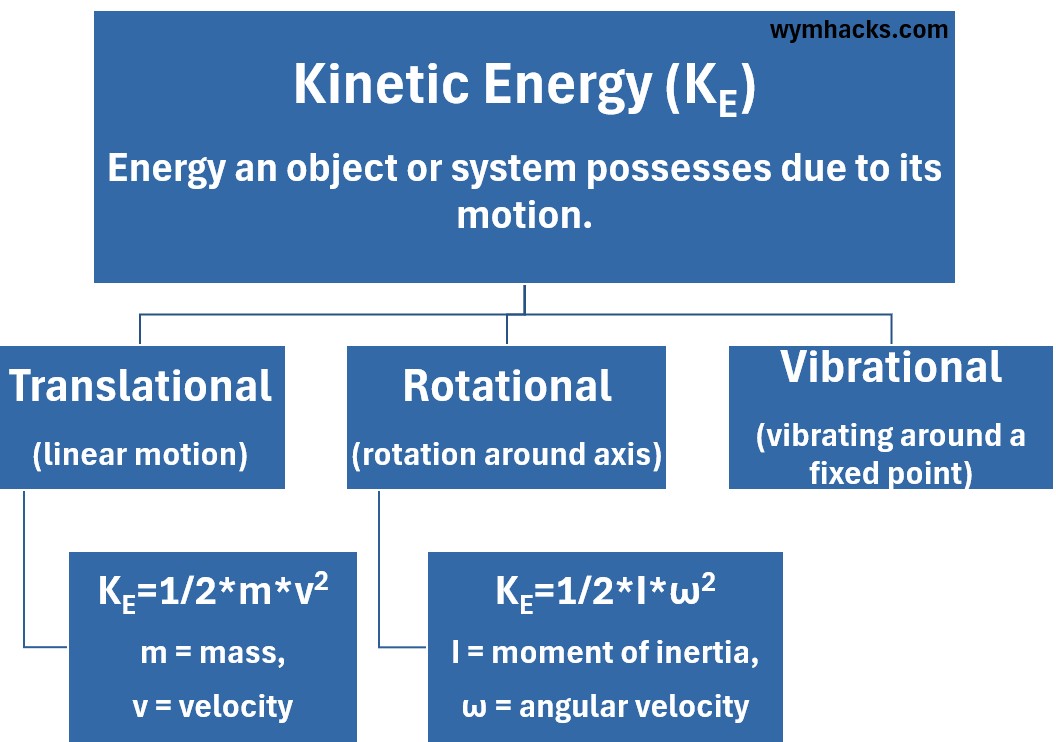
Translational Kinetic Energy
is the energy associated with the linear motion of an object’s center of mass through space,
- such as a car driving down a road or
- a gas molecule moving in a straight line.
- It is the most commonly known form of kinetic energy, calculated using the familiar formula
- KE = (1/2)mv2,
- where m is mass and
- v is velocity.
Rotational Kinetic Energy
is the energy possessed by an object due to its spinning or rotation about an internal axis.
This is present when
- a top spins,
- a wheel turns,
- or a molecule rotates in space,
and it depends on the object’s moment of inertia I (distribution of mass) and its angular velocity ω
- KE = (1/2)Iω2
Potential Energy
Potential energy is stored energy that an object possesses due to its position, structure, or state, giving it the potential to do work.
Examples include
- an object held high above the ground (gravitational potential energy),
- a stretched spring (elastic potential energy),
- or energy stored in chemical bonds (chemical potential energy).
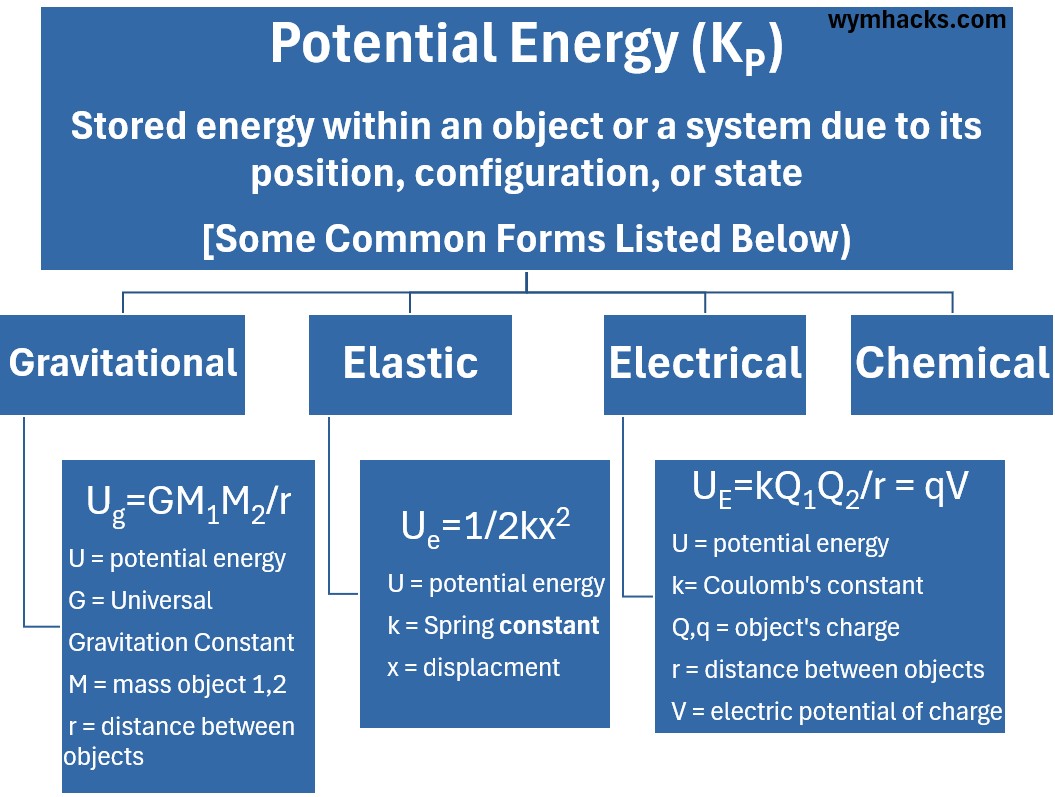
Gravitational Potential Energy
is the energy stored in an object due to its height or vertical position above a reference point, resulting from the gravitational pull.
For example,
- a roller coaster sitting at the top of a hill or
- water held behind a dam has GPE,
which is converted to kinetic energy when it falls.
The general formula for the gravitational potential energy between two point masses, M1 and M2, separated by a distance r is:
Ug = -G M1 M2/r
- U is the gravitational potential energy (in Joules, J).
- G is the Universal Gravitational Constant (G = 6.674E-11 Nm2kg2).
- M1 and M2 are the masses of the two objects (in kilograms, kg).
- r is the distance between the centers of the two masses (in meters, m).
- Ug = mgh is a simplified approximation of the general formula, valid only for
- objects near a planet’s surface where
- the change in height h is very small compared to the planet’s radius.
- This simplification assumes the gravitational force is constant.
- m = the amount of matter in the object being lifted or positioned
- g = the Acceleration Due to Gravity (g = 9.81m/s2 on earth)
Elastic Potential Energy
This is the energy stored in an elastic object when it is stretched, compressed, twisted, or otherwise deformed.
A compressed spring, a stretched rubber band, or a drawn archer’s bow all store this energy, which is released when the object returns to its original shape.
Ue = 1/2kx2
- U is the Elastic Potential Energy: The energy stored in the spring due to its deformation (stretching or compressing) (Unit: Joules).
- k is the Spring Constant: A measure of the stiffness of the spring or elastic object. (Unit: Newtons per meter)
- x is the Displacement: The distance the spring is stretched or compressed from its equilibrium position (its natural, resting length) (Unit: Meters).
Electrical Potential Energy
Electric Potential Energy is the stored energy that a system of charges possesses due to the relative positions of those charges within an electric field.
It’s analogous to gravitational potential energy, where a mass has stored energy due to its position in a gravitational field.
The most fundamental equation for Electrical Potential Energy (UE) describes the energy stored in the configuration of two point charges, Q1 and Q2, separated by a distance r.
UE = kQ1Q2/r
- UE is the Electrical Potential Energy: The energy stored in the electric field due to the relative positions of the two charges.
- It represents the work required to assemble the system of charges (bring them from infinite separation to the distance r). (Unit: Joules).
- k is Coulomb’s Constant (8.99E9 Nm2/C2): A proportionality constant that relates the magnitude of the electrostatic force (and thus the potential energy) to the charges and distance.
- Q1 and Q2 are the Charges: The magnitudes of the two point charges. (Unit: Coulombs)
- r is the Separation Distance : The distance between the centers of the two point charges. (Unit: Meters)
Chemical Potential Energy
This energy is stored within the chemical bonds of atoms and molecules.
When a chemical reaction occurs, such as burning natural gas or digesting food, these bonds are broken and new ones are formed, releasing the stored energy, typically as heat and light (thermal energy).
Food, gasoline, and batteries are common examples.
Chemical potential energy is a form of potential energy because it is stored and ready to be released or absorbed when a chemical reaction occurs, transforming the substance into a new one.
The amount of useful energy available from a chemical reaction is best described by
- ΔG = ΔH – TΔ S (Gibbs-Helmholtz equation, AKA, Gibbs Free Energy Equation)
- change in Gibbs Free Energy (ΔG),
- which combines the effects of heat (ΔH, Enthalpy) and
- disorder (ΔS, Entropy) at a given temperature (T):
A negative ΔG means the reaction is spontaneous (thermodynamically favorable) because the stored chemical potential energy is released (exergonic).
The magnitude (size) of ΔG tells you the maximum amount of useful work the reaction can perform (if negative) or the minimum amount of energy required to make it happen (if positive).
I am barely scraping the surface of an area of science called thermodynamics.
I am including two links below for some excellent video lessons on the topic.
Types and Forms of Energy
We can now look at various types of energy and see where they fit under the fundamental categories of Kinetic or Potential Energy
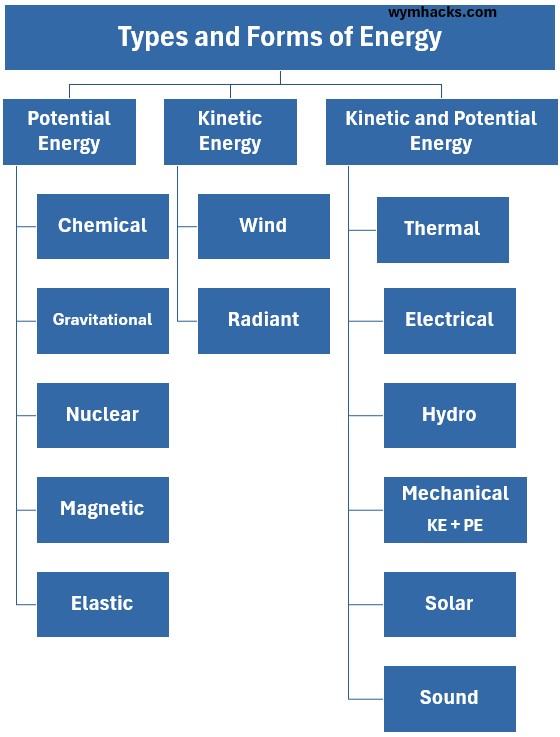
Predominantly Potential Energy Energy Types
- Chemical Energy: Energy stored in the bonds between atoms and molecules.
- It is the energy released during chemical reactions (e.g., burning wood, metabolism of food, or discharging a battery).
- Gravitational Potential Energy: Energy stored due to an object’s height or vertical position within a gravitational field (e.g., water held back by a dam).
- Nuclear Energy: Energy stored in the forces that hold the nucleus of an atom together.
- It is released when atoms are split (fission) or combined (fusion).
- Elastic Potential Energy: Energy stored by the deformation of an elastic object (e.g., a stretched rubber band or a compressed spring)
- Magnetic Potential Energy: It is the stored energy that a magnetic object (like a permanent magnet or a current loop, often modeled as a magnetic dipole) possesses due to its position and orientation within an external magnetic field.
Predominantly Kinetic Energy Energy Types
- Wind Energy: The kinetic energy of moving air masses at a macroscopic scale.
- Radiant Energy: The energy of photons traveling in waves (Electromagnetic Energy).
- Since photons have no rest mass, their energy is defined by their speed
Energy Types That Can Be Either KE and/or PE (State-Dependent)
Thermal Energy: from the random movement and vibration of atoms and molecules.
KE (particle motion) is the dominant effect, but includes PE stored in intermolecular forces.
Electrical Energy: associated with the presence and movement of electric charges.
PE when stored (battery/capacitor) or
KE when flowing as current (moving electrons).
Hydro Energy: The energy of flowing or standing water.
PE (water height in a reservoir)
KE (flowing water/current speed).
Mechanical Energy: The total energy of a macroscopic system, defined as the sum of its kinetic and potential energy.
The sum of an object’s PE (position) and KE (velocity).
Solar Energy: Radiant Energy originating from the Sun.
KE (the light/photons reaching Earth)
PE (captured and stored as chemical or thermal energy).
Sound Energy: Energy transferred by vibrations in a medium (a mechanical wave).
KE (particle velocity) is equally balanced by
PE (elastic compression/rarefaction).
Energy Sources and Conversion Routes to Electricity Generation
The below is such a nice schematic.
It shows the various energy conversion routes that are involved in electricity generation.
Picture: Source: MIT: Energy Sources and Energy Conversion Routes
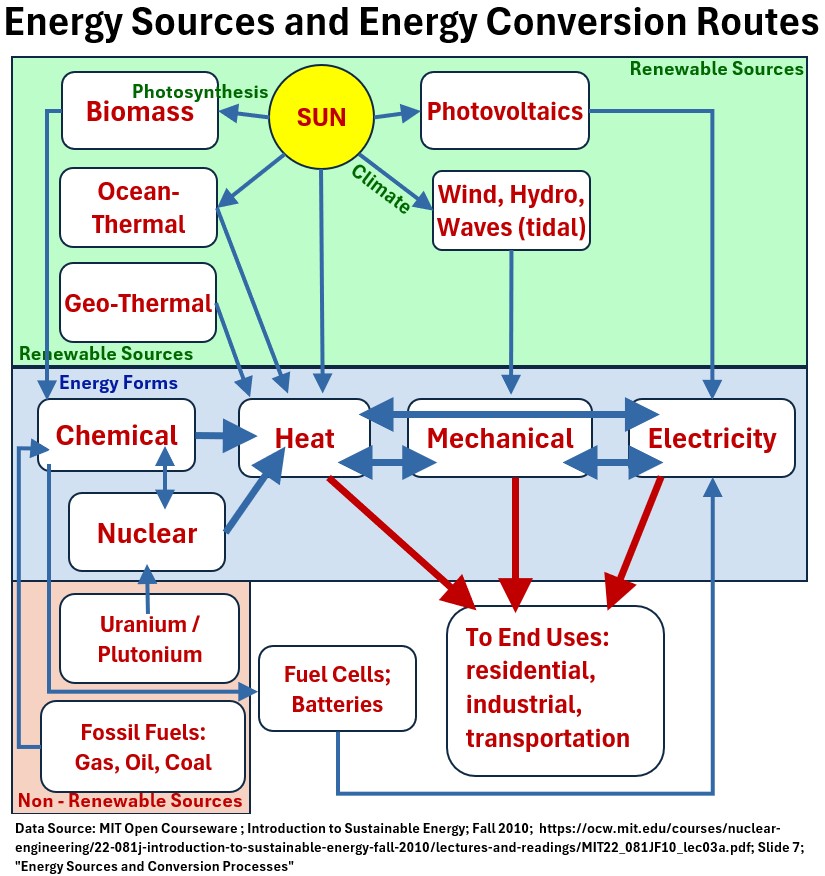
Chemical Energy to Electricity
This describes the standard energy conversion chain used in many conventional power plants (like coal or natural gas plants) to generate electricity.
Chemical Energy → Heat (Thermal Energy)
- Process: Combustion (burning).
- Mechanism: Fuel (e.g., coal, natural gas, oil) reacts rapidly with oxygen.
- This breaks the chemical bonds within the fuel molecules (releasing the stored Potential Energy) and forms new, more stable compounds (like CO2 and Water)
- Result: The excess energy from the reaction is released primarily as Thermal Energy (heat).
Heat (Thermal Energy) → Mechanical Energy
- Process: Boiling/Expansion (using a heat engine).
- Mechanism:
- The heat generated from combustion is used to boil water, creating high-pressure steam.
- The thermal energy is converted into the Kinetic Energy of the steam, which rapidly expands.
- Result: This expanding steam is directed against the blades of a turbine, causing the turbine shaft to rotate.
- This rotational motion is Mechanical Energy.
Mechanical Energy → Electrical Energy
- Process: Electromagnetic Induction (using a generator).
- Mechanism: The rotating turbine shaft is connected to a generator.
- The generator uses the continuous mechanical motion to spin an electromagnet (or conducting coil) within a stationary coil of wire.
- This relative motion induces a flow of electrons.
- Result: The mechanical energy is converted into Electrical Energy (current), which is then transmitted to the grid.
Nuclear Energy to Electricity
The conversion of Nuclear Energy into Electrical Energy uses the exact same conversion chain as the chemical process (coal, natural gas) but substitutes the combustion step with nuclear fission.
It is essentially a specialized heat engine.
Nuclear Energy → Heat (Thermal Energy)
- Process: Fission (splitting of atoms).
- Mechanism: Fuel pellets (typically uranium-235) are bombarded with neutrons inside the reactor core.
- This causes the uranium nucleus to split, releasing an enormous amount of energy, primarily in the form of Kinetic Energy of the fission products and neutrons.
- Result: This kinetic energy quickly translates into intense Heat (Thermal Energy) within the reactor core, heating the circulating coolant (usually water).
Heat (Thermal Energy) → Mechanical Energy
- Process: Steam Generation and Expansion.
- Mechanism: The heat from the reactor core is transferred to water (often in a separate loop to contain radioactivity), turning it into high-pressure, high-temperature steam.
- Result: The expanding steam’s Thermal Kinetic Energy is directed into a large turbine, causing the shaft to rotate.
- This rotational motion is Mechanical Energy
Mechanical Energy → Electrical Energy
- Process: Electromagnetic Induction (using a generator).
- Mechanism: The rotating turbine shaft is connected to a generator.
- The mechanical motion is used to spin magnetic fields around conductive coils.
- Result: This relative movement induces a current, converting the mechanical energy into usable Electrical Energy.
Sun Energy to Wind/Hydro/Tidal to Electricity
Sun → Wind/Hydro/Tidal
The initial step involves the Sun driving Earth’s cycles, storing Kinetic and Potential Energy in natural systems.
Wind
- Conversion: The Sun’s uneven heating of the Earth’s atmosphere creates temperature and pressure differences, causing air masses to move
- Fundamental State: Kinetic Energy of moving air
Hydro
- Conversion: Solar heat causes water to evaporate (Kinetic Energy), which rises and cools to fall as rain/snow at higher elevations (storing Potential Energy), forming rivers.
- Fundamental State: Potential Energy (height) → Kinetic Energy (flow).
Tidal (indirectly related to Sun)
- Conversion: (Indirectly related to the Sun): While primarily driven by the Moon’s gravity, the Sun’s gravitational pull and the Earth’s rotation influence the tides, creating a predictable rise and fall of water.
- Fundamental State: Potential Energy (water height difference).
→ Mechanical Energy
This is the capture step, where the energy of the natural system is converted into the rotation needed to do work.
- Wind: The kinetic energy of the wind pushes against the large blades of a turbine, causing the central shaft to rotate.
- Hydro: The potential energy of water (in a reservoir) converts to kinetic energy as it falls through a pipe (penstock) and strikes the blades of a water turbine, causing the shaft to rotate.
- Tidal: The force of the moving, high-volume water pushes against specialized turbines (similar to hydro) anchored in the current, causing a shaft to rotate.
In all cases, the output of this step is rotational motion, which is a form of Mechanical Energy.
→ Electrical Energy
The final step is uniform across all these renewable technologies.
- Process: Electromagnetic Induction (using a generator).
- Mechanism: The rotating shaft (Mechanical Energy) is connected to a generator. Inside the generator, this motion spins magnetic fields around stationary wires, which induces a flow of electrons (current).
- Result: The mechanical energy is converted into usable Electrical Energy that is transmitted to the power grid.
This conversion chain is highly efficient because it bypasses the initial thermal (heat) stage used by fossil fuel and nuclear power.
Sun to Photovoltaics to Electricity
This conversion process, used in solar panels, is one of the most direct and elegant ways to generate electricity, bypassing the intermediate mechanical and thermal stages.
The conversion chain is: Radiant Energy (KE) → Electrical Energy.
Sun → Photovoltaics (PV)
Source: Radiant Energy (specifically, photons of light) emitted by the sun. This is a form of Kinetic Energy (KE).
- Process: The Photovoltaic Effect.
- Mechanism: A solar panel is made of photovoltaic cells, which contain layers of semiconductor material (most commonly silicon).
- A key feature of these layers is the creation of an internal electric field across a junction (the p-n junction).
- When a photon hits the cell with enough energy, it is absorbed by a silicon atom.
- The photon’s energy is transferred to an electron, which is then knocked loose from its atomic bond.
- The internal electric field immediately forces this freed electron into a specific direction, pushing it to the n-type layer.
- Result: The flow of these freed electrons constitutes an electric current.
Photovoltaics (PV) → Electricity
- Process: Current Flow (Direct Conversion).
- Mechanism: The continuous generation of a directional flow of electrons within the solar cell creates Direct Current (DC) electricity.
- Conversion (Inverter): Since most homes and the electrical grid use Alternating Current (AC), the DC power generated by the solar panels must pass through an inverter.
- The inverter converts the DC power into usable AC power.
- Result: Usable Electrical Energy that can power devices or be fed into the utility grid.
This process is a single-step conversion from one form of Kinetic Energy (Radiant KE) directly to another form of Kinetic Energy (Electrical Current KE), making it highly efficient.
Sun to Biomass to Heat Conversion
This describes the ancient and continuous energy conversion chain used by nature and harnessed by humans through the burning of plant matter (biomass) for heat.
The conversion chain is: Radiant Energy (KE) → Chemical Energy (PE) → Heat (Thermal Energy)
Sun → Biomass (Chemical Energy)
- Process: Photosynthesis.
- Mechanism: Plants absorb Radiant Energy (photons/light) from the sun.
- Using water (H2O) and carbon dioxide (CO2), they convert the radiant kinetic energy into stored Chemical Potential Energy in the form of complex organic molecules (sugars, cellulose, lignin).
- Result: The plant’s structure (biomass—wood, leaves, algae, etc.) becomes a form of concentrated Chemical Energy
Biomass (Chemical Energy) → Heat (Thermal Energy)
- Process: Combustion (burning) or Biological Oxidation.
- Mechanism: When biomass is burned, the stored chemical bonds are rapidly broken in a reaction with oxygen. This process releases the captured potential energy.
- Result: The released energy manifests primarily as Heat (Thermal Energy), often accompanied by light (Radiant Energy).
This heat can be used directly for cooking or space heating (or commercial applications as well).
This conversion chain is one of the foundational cycles of life on Earth, showing how the sun’s energy is temporarily stored before being released back into the environment as heat.
Note that the process of converting Sun → Biomass → Heat is the first, critical stage in the creation of fossil fuels.
Fossil fuels are essentially ancient, highly concentrated biomass that has undergone immense changes over geological time.
Energy Conversion Efficiencies for Various Technologies
Below is a bar graph showing conversion efficiencies for various conversion sequences.
Efficiency is simply the percent of the incoming energy that is being used for useful work.
These are probably not the latest values but they give you a nice reference point for how different some technologies are in terms of efficiency.
For example, Solar Power is 15% efficient compared to 90% for hydro power!
Graph: Energy Conversion Efficiencies for Various Technologies
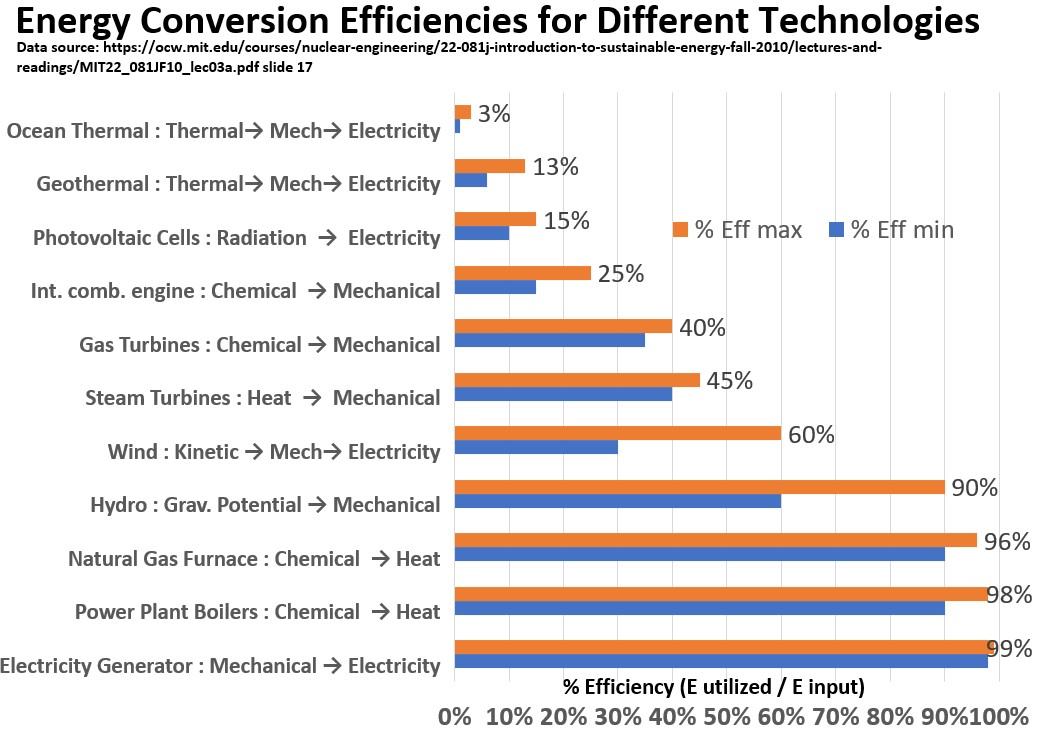
Observations based on the Graph Above:
- The energy-conversion-to-useful-work percentages (called exergy) cover a wide range from the low teens all the way to close to 100%
- Chemical (fuel) to Thermal (steam) conversion efficiency can be high (90%)
- Thermal to Mechanical energy conversion efficiency is much lower at < 50%
- Mechanical to Electrical energy conversion efficiency can be very high (98%+)
- Potential Energy Conversion (Hydro) to electricity is very high (90%)
- Combining relatively inefficient elements of a steam/power cycle results in a fairly high conversion efficiency
It’s amazing that the turbines (Gas and Steam) that are the workhorses of our power generation infrastructure have pretty low efficiencies (45%) although by combining some of the equipment we can actually increase the efficiency by 33%.
What is the main learning here? When it comes to power plant efficiency, in the real world, you cant win or even break even.
- i.e. a power plant that operates at 60% efficiency is going to be best in class.
- In the US and Europe the average efficiency of power plants is probably more in the range of 40 to 50%.
- That is, for every 100 BTUs , we can extract roughly 50 BTU for useful work.
Appendix 1 – Energy Type and PE and/or KE Categorization
Chemical Energy (Potential Energy)
Microscopic Chemical Potential Energy:
- Chemical energy is stored in the bonds between atoms and molecules.
- Arises from the electrostatic forces (a form of potential energy) between electrons and nuclei.
- When chemical reactions occur, these bonds are rearranged, releasing or absorbing energy as the system moves to a lower or higher energy configuration.
- It’s essentially microscopic potential energy.
Electrical Energy (Potential & Kinetic)
Electrical_Potential Energy:
- Stored energy due to the position or separation of electric charges within an electric field.
- Examples include the energy stored in a charged capacitor (voltage) or the energy a charge possesses due to its position relative to other charges.
Electrical_Kinetic Energy:
- The energy of moving electric charges (electric current).
- For example, electrons flowing through a wire constitute kinetic energy.
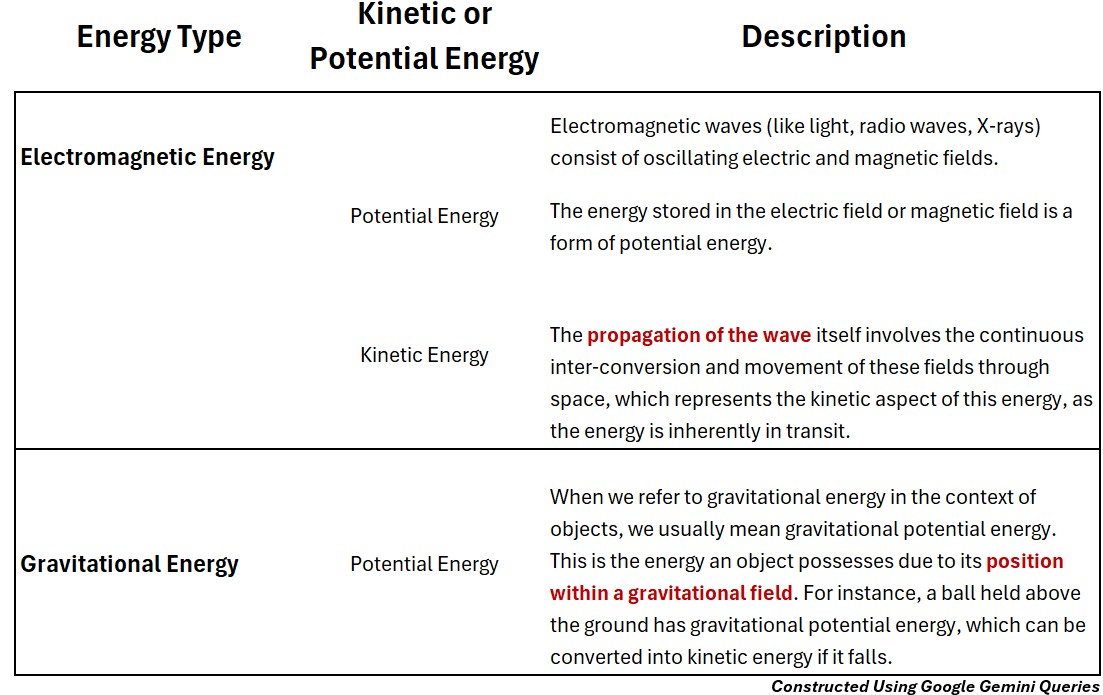
Electromagnetic Energy (Potential & Kinetic)
Electromagnetic waves (like light, radio waves, X-rays) consist of oscillating electric and magnetic fields.
Electromagnetic_Potential Energy
The energy stored in the electric field or magnetic field is a form of potential energy.
Electromagnetic Kinetic Energy
The propagation of the wave itself involves the continuous inter-conversion and movement of these fields through space, which represents the kinetic aspect of this energy, as the energy is inherently in transit.
Gravitational Energy (Potential Energy)
Gravitational Potential Energy
- When we refer to gravitational energy in the context of objects, we usually mean gravitational potential energy.
- This is the energy an object possesses due to its position within a gravitational field.
- For instance, a ball held above the ground has gravitational potential energy, which can be converted into kinetic energy if it falls.
Hydro Energy (Potential & Kinetic)
Macroscopic Hydro Potential Energy
Water stored at a height (e.g., behind a dam in a reservoir) possesses gravitational potential energy.
Macroscopic Hydro Kinetic Energy
As this water flows downwards and gains speed (e.g., through a turbine), its potential energy is converted into macroscopic kinetic energy.
Magnetic Energy (Potential Energy)
Magnetic energy is energy stored in a magnetic field.
- This energy is associated with the configuration of magnetic dipoles or currents that create the field.
- For example, the energy stored in an inductor when a current flows through it is magnetic potential energy.
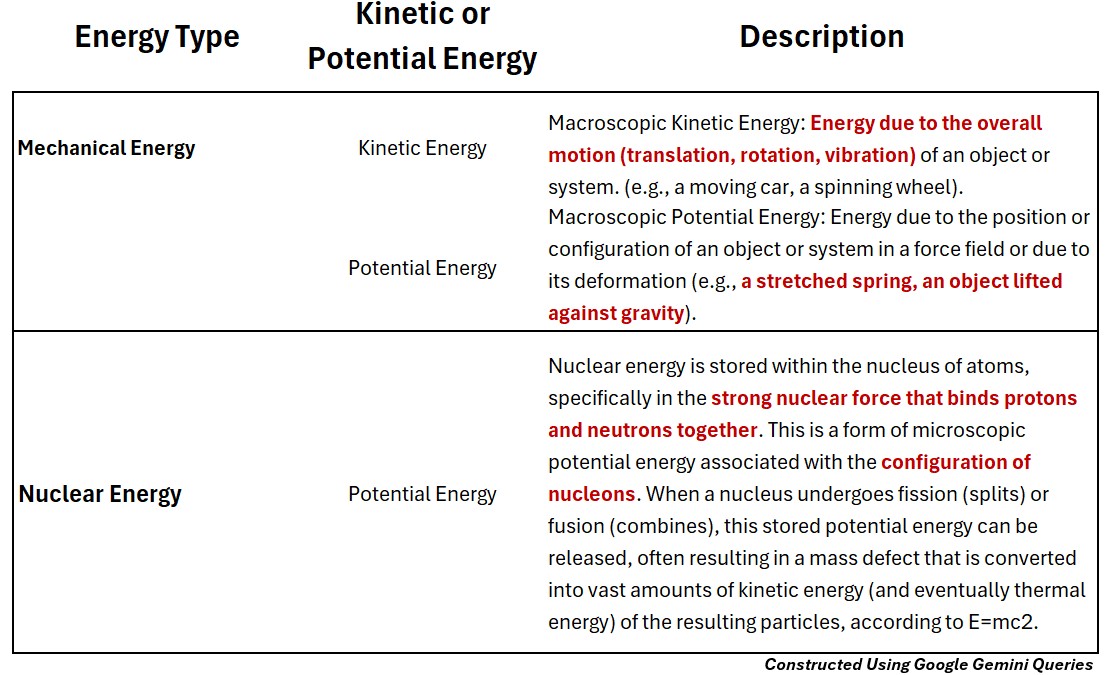
Mechanical Energy (Potential & Kinetic)
Macroscopic Mechanical Kinetic Energy
Energy due to the overall motion (translation, rotation, vibration) of an object or system. (e.g., a moving car, a spinning wheel).
Macroscopic Mechanical Potential Energy
Energy due to the position or configuration of an object or system in a force field or due to its deformation (e.g., a stretched spring, an object lifted against gravity).
Nuclear Energy (Potential Energy)
Microscopic Nuclear Potential Energy
- is stored within the nucleus of atoms, specifically in the strong nuclear force that binds protons and neutrons together.
- This is a form of microscopic potential energy associated with the configuration of nucleons.
- When a nucleus undergoes fission (splits) or fusion (combines), this stored potential energy can be released, often resulting in a mass defect that is converted into vast amounts of kinetic energy (and eventually thermal energy) of the resulting particles, according to E=mc2.”
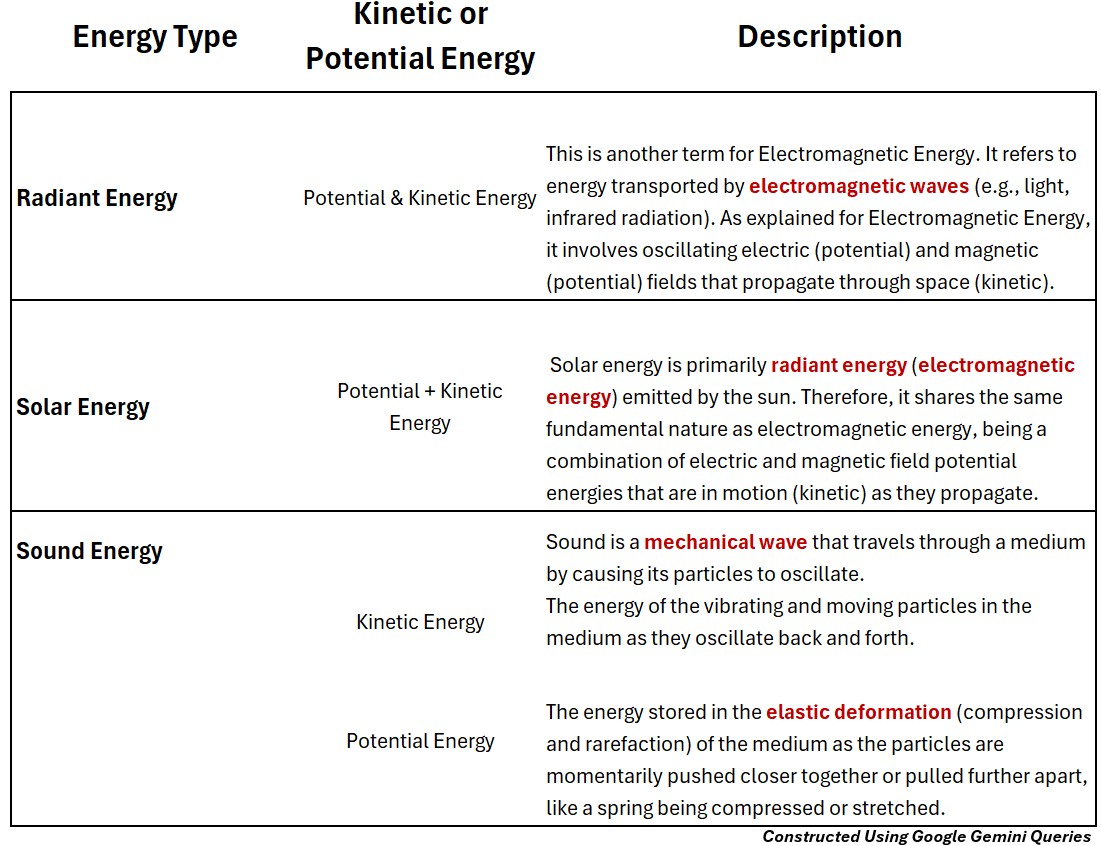
Radiant Energy (Potential and Kinetic)
Potential and Kinetic Radiant Energy
This is another term for Electromagnetic Energy.
- It refers to energy transported by electromagnetic waves (e.g., light, infrared radiation).
- As explained for Electromagnetic Energy, it involves oscillating electric (potential) and magnetic (potential) fields that propagate through space (kinetic).
Solar Energy (Potential and Kinetic)
Solar_Potential and Kinetic Energy
- Solar energy is primarily radiant energy (electromagnetic energy) emitted by the sun.
- Therefore, it shares the same fundamental nature as electromagnetic energy, being a combination of electric and magnetic field potential energies that are in motion (kinetic) as they propagate.”
Sound Energy (Potential & Kinetic)
Sound is a mechanical wave that travels through a medium by causing its particles to oscillate.
Sound_Kinetic Energy
The energy of the vibrating and moving particles in the medium as they oscillate back and forth.
Sound Potential Energy
The energy stored in the elastic deformation (compression and rarefaction) of the medium as the particles are momentarily pushed closer together or pulled further apart, like a spring being compressed or stretched.
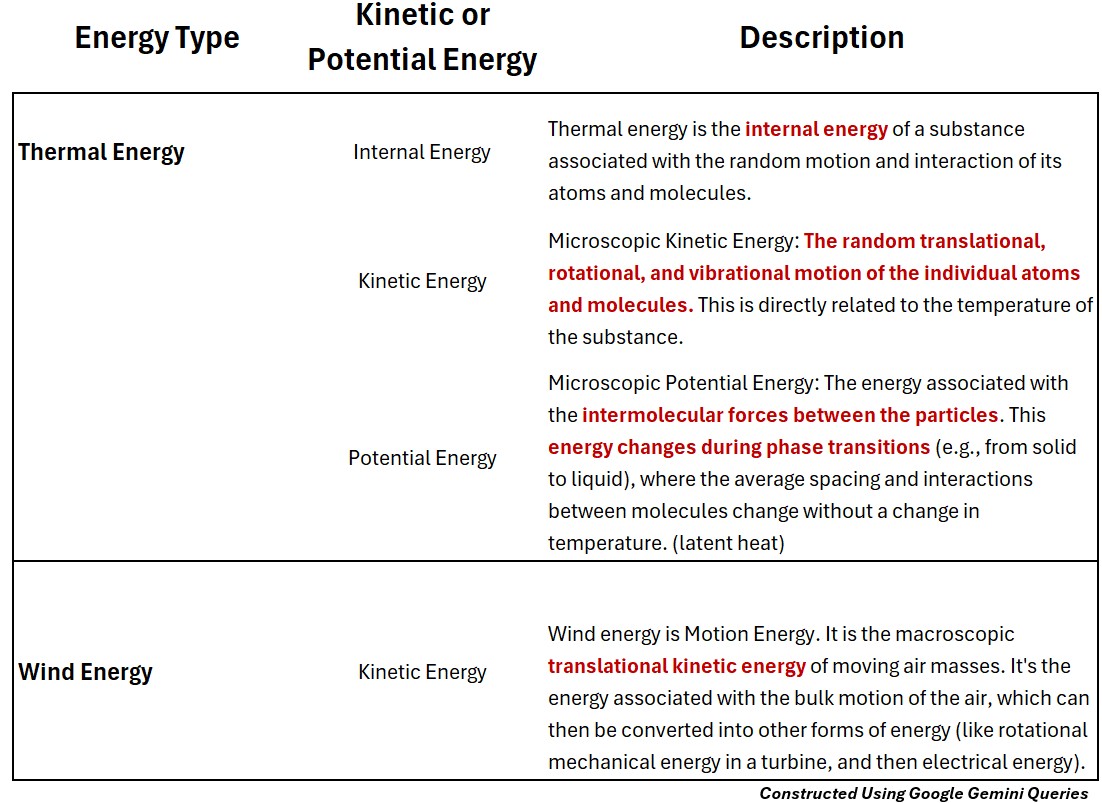
Thermal Energy (Potential and Kinetic)
Thermal energy is the internal energy of a substance associated with the random motion and interaction of its atoms and molecules.
Microscopic Thermal Kinetic Energy
The random translational, rotational, and vibrational motion of the individual atoms and molecules. This is directly related to the temperature of the substance.
Microscopic Thermal Potential Energy
The energy associated with the intermolecular forces between the particles. This energy changes during phase transitions (e.g., from solid to liquid), where the average spacing and interactions between molecules change without a change in temperature. (latent heat)
Wind Energy (Kinetic)
Macroscopic Kinetic Wind Energy
Wind energy is Motion Energy.
- It is the macroscopic translational kinetic energy of moving air masses.
- It’s the energy associated with the bulk motion of the air, which can then be converted into other forms of energy (like rotational mechanical energy in a turbine, and then electrical energy).
Appendix 2 – Energy Type and PE and/or KE Categorization (Tables)

Electromagnetic and Gravitational Energy
Hydro and Magnetic Energy

Mechanical and Nuclear Energy
Radiant, Solar and Sound Energy
Thermal and Wind Energy
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.
