Menu (linked Index)
Fundamental Particles and Forces
Last Update: June 28, 2025
Introduction
This post describes the elementary particles of the Standard Model of particle physics as well as the four fundamental forces.
The Standard Model is not complete in that the theorized force carriers of the gravitational force (Gravitons) have not been discovered yet.
- So the model only covers three of the four fundamental forces
- The Strong Force
- The Weak Force
- The Electromagnetic Force
- and is therefore sometimes described as the theory of almost everything.
For completeness we’ll cover the Gravitational Force as well.
Here are some of the references/sources I used to put this together.
- The Standard Model – CERN
- Fundamental Particles – Bozeman Science
- Four Fundamental Forces – Khanacademy.org
- The Four Fundamental Forces of Nature-Arvin Ash
- BBC Bitesize: The Standard Model
- Klonusk: All Fundamental Forces and Particles Explained Simply
- The “God Particle”: a story of Big Science, Big Data, and Human Ingenuity
- Google Gemini
I barely scrape the surface of this topic so use the internet, your bookstore or your library if you need to dig deeper into any particular topic.
I recommend that you watch the Klonusk video a few times and then expand your effort from there if any particular subtopics pique your interest.
Zooming In
Fundamental or Elementary particles are the indivisible (as far as we know today) building blocks of matter.
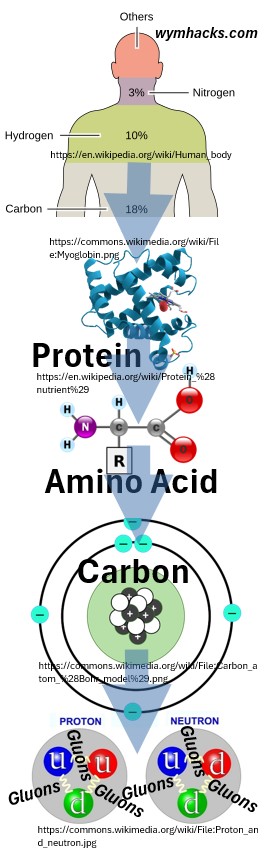
Let’s start with a human body to begin our journey downward to the smallest particles.
Adult males and females have about 36 Trillion and 28 Trillion cells respectively.
- Remember 1 trillion = 1,000,000,000,000 = 1E12 = 1 x 1012
Each cell has roughly 100 Trillion atoms.
- That’s 100,000,000,000,000 = 100E12 = 100 x 1012
Consider the schematic below.
It indicates that carbon atoms make up about 18% of a human.
For example, a lot of these carbons make up amino acid molecules which in turn make up proteins.
Picture: From Human to Quark
A Carbon Atom
If we look at one carbon atom from one amino acid molecule, we might see a simple representation as shown above.
In reality the nucleus (the center made of protons and neutrons) is a tiny spec compared to the total size of the atom.
Some characteristics of this carbon atom are:
- The nucleus of the atom is made up of 6 neutrons and 6 protons.
- The nucleus radius is around 2.7 femtometers (2.7E-15)
- The atom’s total radius is around 70 picometers.(70E-12)
- So, the radius of the atom is about (70E-12)/(2.7E-15) = 25,926 meters x the radius of the nucleus at its center.
- That means if the carbon atom was the size of a basketball (about a .12 m radius),
- the radius of the carbon atom would be about 3.1 km (1.9 miles)
Picture: Carbon Atom with a Nucleus Enlarged to the Size of a Basketball
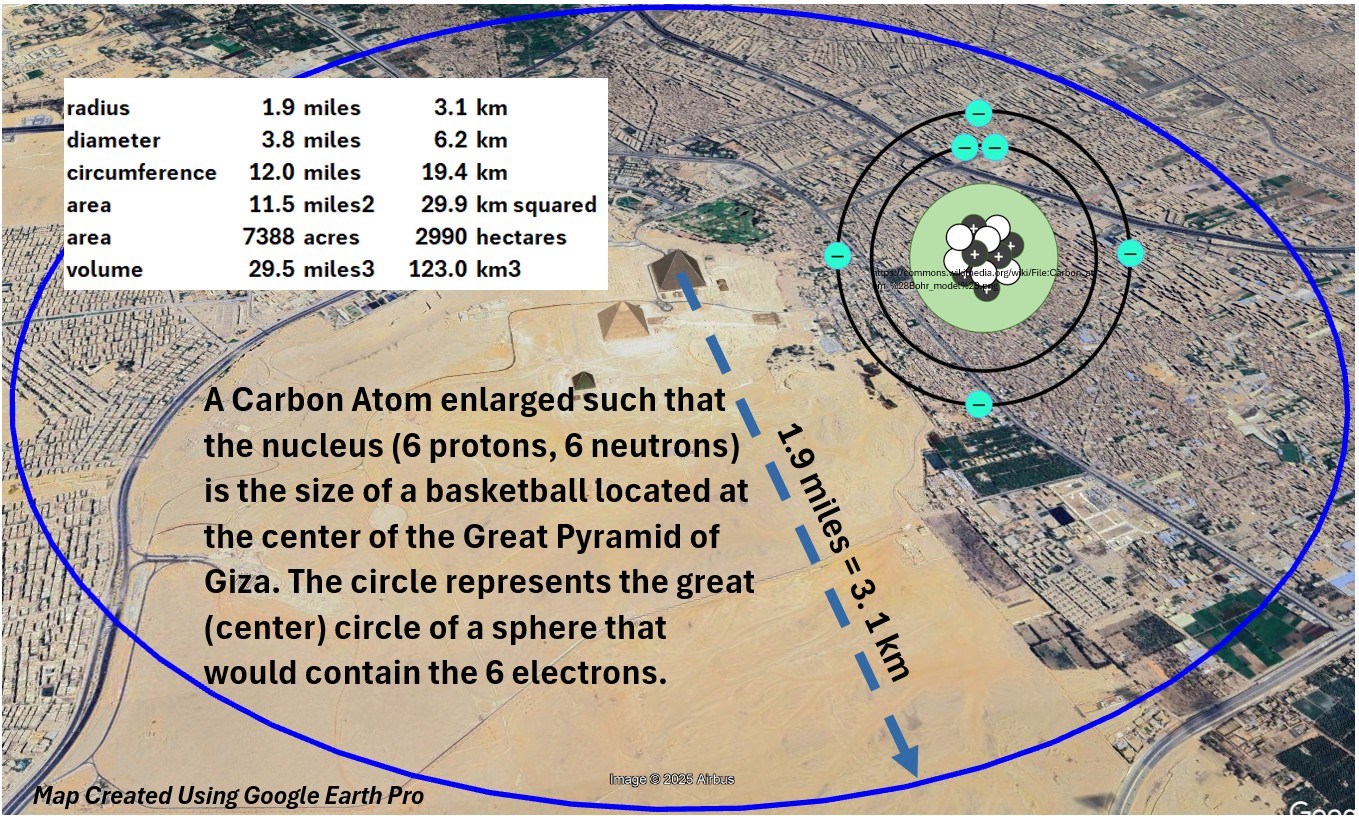
- So on an atomic level, there is a vast space between the outer edges of the carbon atom and its nucleus
- There are 6 electrons buzzing around in that vast space around the carbon nucleus
- you can describe this space as a probability cloud
- That tiny nucleus in the center of the atom consists of
- 6 protons (positively charged)
- 6 neutrons (with no charge)
- Each proton (and neutron) will weigh about 1.67E-27 kg = 1.67 x 10-27 kg
- That’s .00000000000000000000000000167 kg (i.e. very f……g small).
- Each electron will be even lighter at 9.109 x 10-31 kg
- meaning protons or neutrons are about 1,833 x heavier than each electron
Deeper
These electrons and protons and neutrons in the carbon atom are subatomic particles but only the electrons would be considered fundamental particles.
The drawing above shows, in the final step, that neutrons and protons are made up of even smaller, elementary particles called quarks.
So, electrons and quarks are elementary (fundamental) particles, but there are more.
The Standard Model
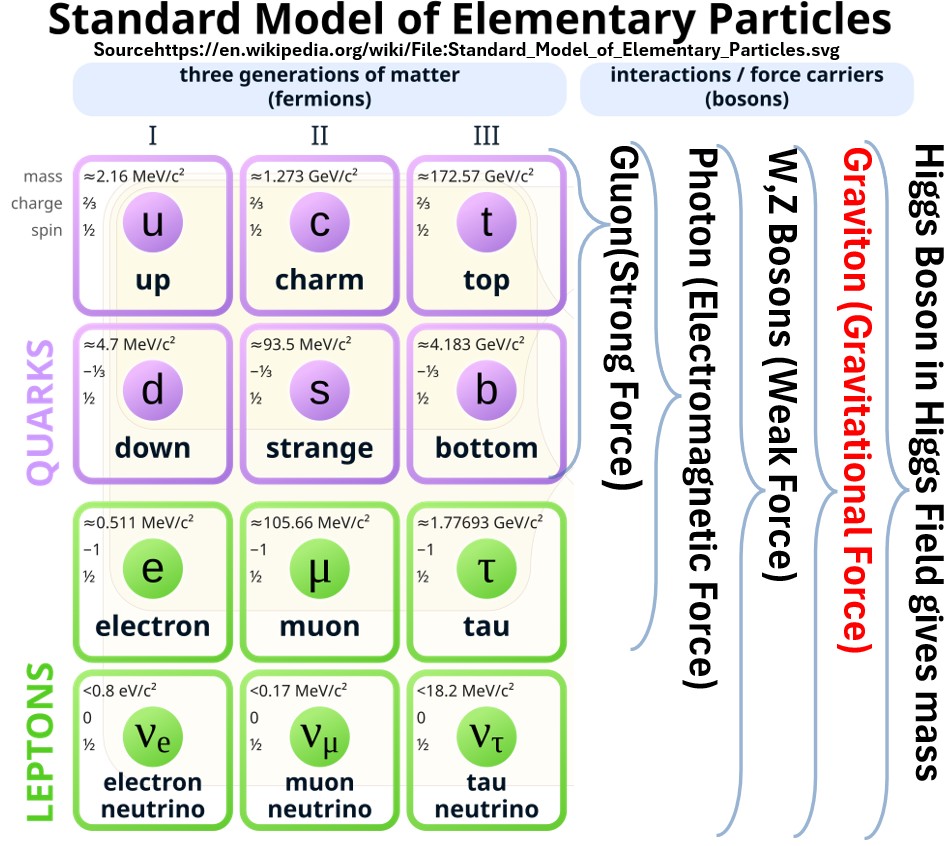
The Standard Model in particle physics
- describes the fundamental particles (6 quarks, 6 leptons, and their antimatter counterparts) and
- three of the four fundamental forces (strong, weak, and electromagnetic) that govern how they interact,
- all mediated by force-carrying particles (bosons)
- and with the Higgs boson providing mass.
- It notably excludes gravity.
All elementary (fundamental) particles have three basic properties:
mass
- Recall that E = mc2
- E = Energy; m = mass; c= speed of light = 299,972,458 m/s
- The mass of an electron is about 9.10938E-31 kg
- So E for an electron = (9.10938E-31 kg)(299,792,458 m/s)2 = 8.18710577547575E-14 joules
- E = (8.18710577547575E-14 joules)(1 eV/1.60E-19 J)(M/1E6) = .511 MeV = .511 mega electron volts
- The mass of an electron in E/c2 = .511 MeV/c2
- Compare this to the mass of a 70 kg human: 3.93E+31 MeV/c2
charge
Charge is an intrinsic property of an elementary particle that determines how it interacts with the electromagnetic force.
It’s a fixed, quantized value for each particle type.
An electron always has a charge of -1, a proton +1, and
quarks have fractional charges like +2/3 or -1/3
Particles with charge create and respond to electric and magnetic fields.
- spin
- Spin is an intrinsic property of elementary particles and is related to angular momentum.
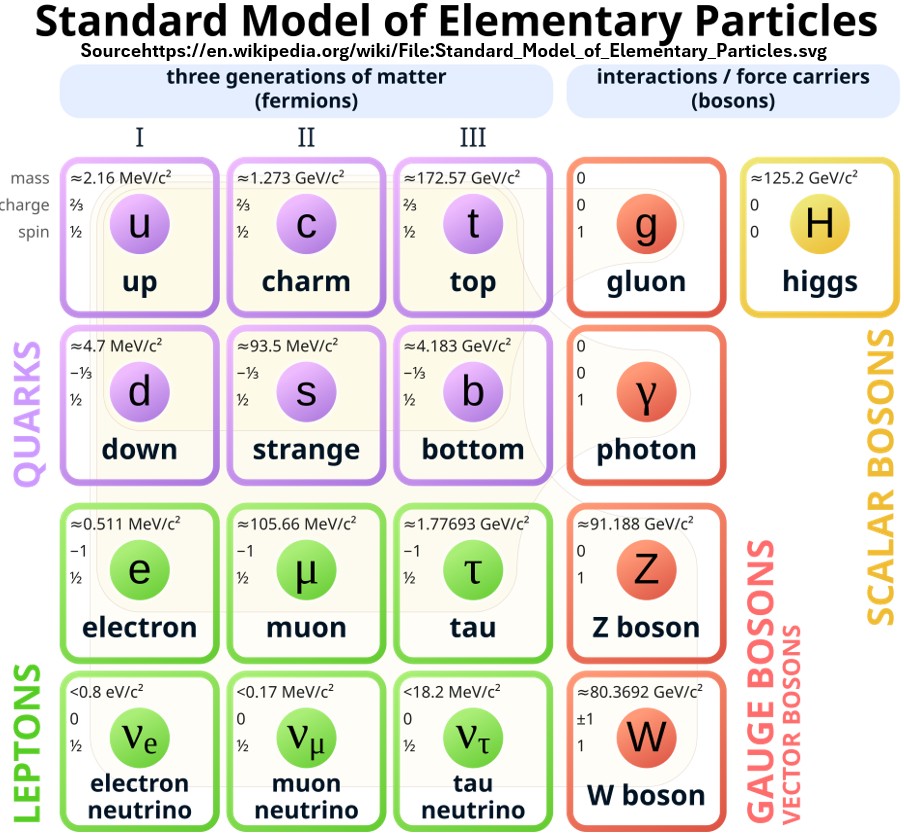
Chart: Standard Model of Elementary Particles (source: Wikipedia)
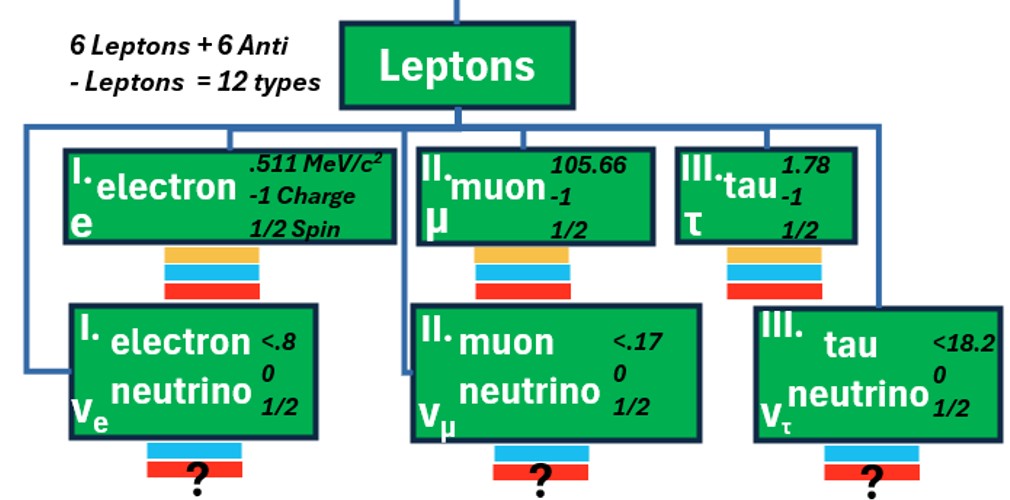
The 12 Fermions Are The “Matter Particles”
- Fermions are categorized as either quarks or leptons.
- Quarks are always grouped.
- Quarks make up baryons e.g. neutrons and protons and mesons.
- Mesons and baryons are both called hadrons.
- The strong fundamental force, via gluons, keeps quarks together (and therefore neutrons and protons)
- Leptons are loners with their most famous representative being the electron.
- Leptons interact via the weak force via photons
- More on these later.
- Fermions are the building blocks of matter.
- e.g. Protons and neutrons are made of quarks.
- They are categorized into three generations (I, II, or III) of pairs
- e.g. Generation I. up and down quarks.
- Fermions all have 1/2 spins
- Spin is an inherent property of the particle, much like its mass or electric charge.
- Spin is quantized and takes on specific, discrete integer or 1/2 integer values.
- Spin behaves like angular momentum.
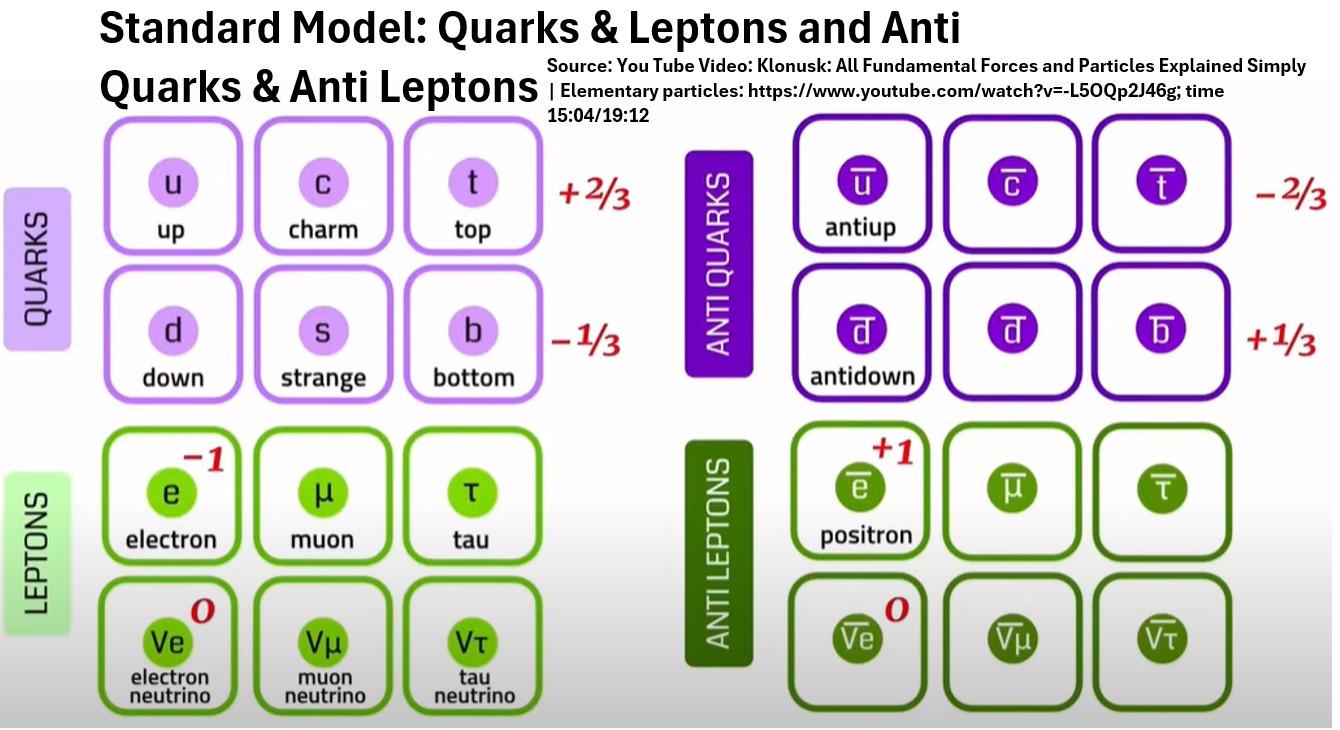
- There are 12 additional antiparticles associated with each fermion.
- They are roughly the same except they carry opposite charges
Chart: Quarks, Anti Quarks, Leptons, Anti Leptons (Source: Klonusk)
The 5 bosons Are Also Fundamental Particles
- Bosons are energy carriers (the Gauge Bosons) and mass givers (Higgs Boson).
- Fermions interact by exchanging Gauge Bosons.
- Bosons are mainly responsible for mediating the fundamental forces between particles.
- Gauge Bosons bind matter together (e.g. quarks in a single proton and protons and neutrons in a nucleus)
- Gauge Bosons can repel fermions as well (e.g. 2 electrons repel each other)
- The Higgs Boson gives fundamental particles their mass (and interacts with the Higgs Field).
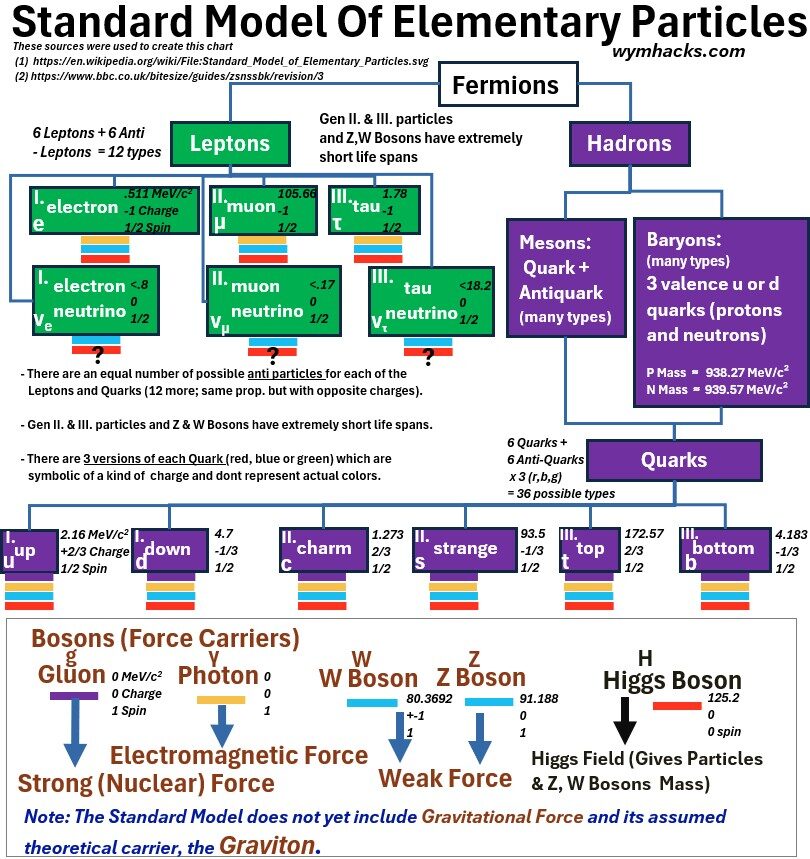
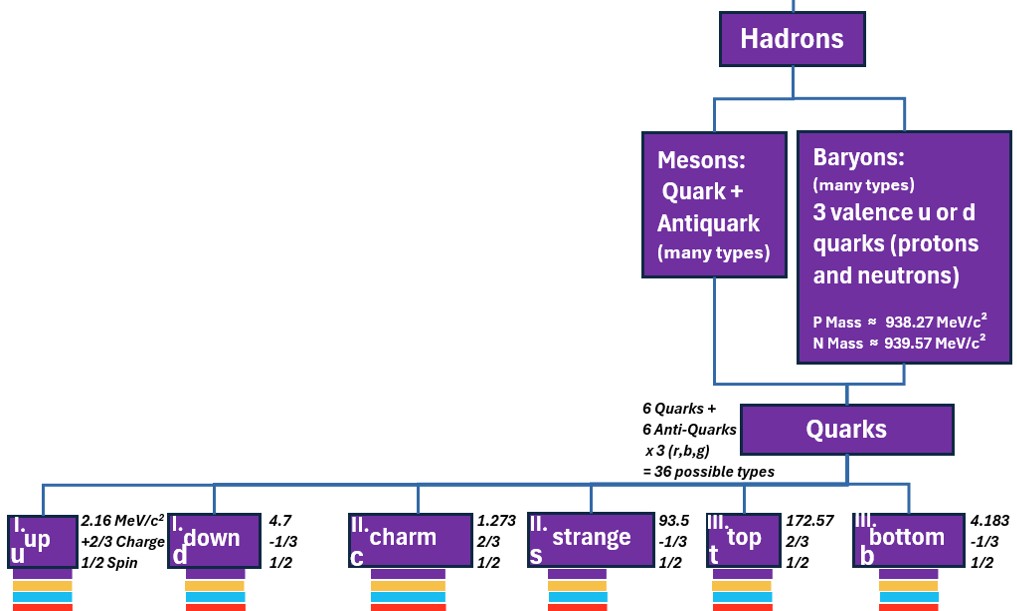
Below is a more detailed Standard Model schematic showing the hierarchy of terminology a little more clearly including where the terms hadron, meson, and baryon fit.
Chart: Standard Model of Elementary Particles Showing Hierarchy of Terms
In the chart above,
- the color bars under each particle indicate which boson interactions exist.
- hadrons are combinations of quarks, and
- baryons are hadrons where the most “property influencing” quarks come in groups of three.
- neutrons and protons are baryons
- these quarks are sometimes described as valence quarks indicating there are other particles but these are the main ones.
- The force carrying bosons are associated with various fundamental forces
- The gluon is the force carrier for the strong force (keeps the nucleus together).
- The photon is the force carrier for the electromagnetic force.
- The W and Z Bosons are the force carriers of the weak force.
- Physicists would love to unify the Standard Model and incorporate the theoretical yet undiscovered gravitational force carrier, the graviton, but so far this has not been discovered.
We have to talk more about quarks!
Quarks
The quark model was independently proposed by physicists Murray Gell-Mann and George Zweig in 1964.
According to Gell-Mann, he had a sound in mind but wasn’t sure how he wanted to spell it until he discovered the following passage in James Joyce’s Finnegan’s Wake:
- Three quarks for Muster Mark!
- Sure he has not got much of a bark
- And sure any he has it’s all beside the mark.
- But O, Wreneagle Almighty, wouldn’t un be a sky of a lark
- To see that old buzzard whooping about for uns shirt in the dark
- And he hunting round for uns speckled trousers around by Palmerstown Park?
– James Joyce, Finnegan’s Wake
Weird.
I provide two charts below showing the three generations of quarks.
I like the second one a little better, though, because it also shows that baryons and mesons are collections of quarks.
Chart: Three Generations of Quark Particles Excerpted from Wikipedia
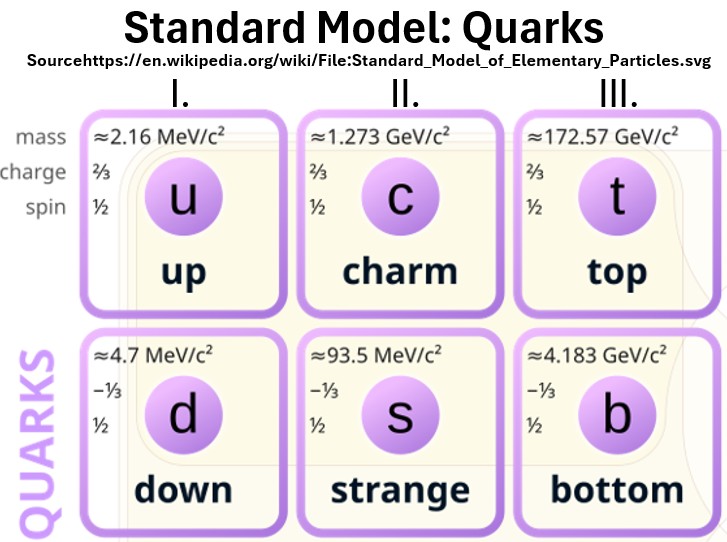
Chart: Quarks (Hadrons, Mesons, Baryons)
Hadrons
Quarks don’t exist individually. They come in combinations.
- Up and down quarks (I. generation) are long lived.
- Gen II and III quarks (e.g. charm, strange, top, and bottom) are very short lived (much less than a second).
Combinations of quarks can produce mesons and baryons of which there are many.
Mesons and baryons are both types of hadrons.
Hadrons are composite particles that are held together by the strong nuclear force (via force carriers called gluons).
- Mesons consist of a quark and an antiquark, while
- Baryons are made of three quarks.
While a vast variety of various mesons and baryons can be created in high-energy environments, almost all of these are highly unstable and rapidly decay.
Protons and neutrons, both baryons, stand out as the only stable or long-lived hadrons under normal conditions making them
- the foundational building blocks of atomic nuclei and, consequently,
- the vast majority of visible matter in the universe today.
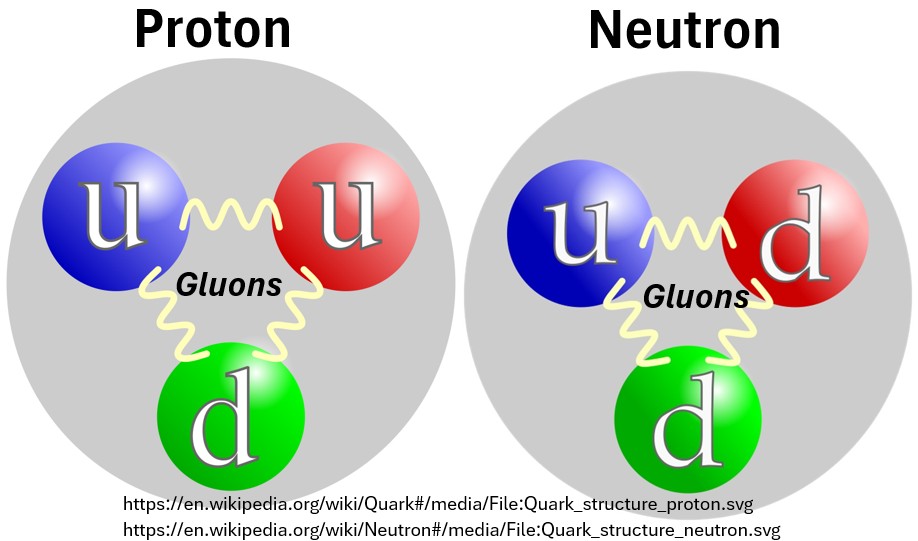
Baryons
Every proton and neutron in every atom (and they are all the same) is made up of a Baryon.
- Protons and Neutrons primarily get their structure and stability from a composite of three quarks.
- A proton comprises two up quarks and one down quark (uud)
- The sum of their charges produces the net charge of a proton.
- i.e. up quark (2/3 charge) + up quark (2/3 charge) + down quark (-1/3 charge) = +1
- A neutron comprises two down quarks and one up quark (ddu)
- The sum of their charges produces the net charge of a neutron.
- i.e. down quark (-1/3 charge) + down quark (-1/3 charge) + up quark (2/3 charge) = 0 charge
Picture: Quarks and Gluons in Protons and Neutrons
Even though baryons are the “big dogs” (sometimes referred to as Valence Quarks) inside a proton or neutron they are not the only sub particles.
- In fact, there are many other quark and anti quark combinations that come into and out of existence.
- The reality inside a proton (or any hadron) is far more dynamic and complex than just three valence quarks.
This is where the concept of the “sea” of virtual particles comes in, and this “sea” includes mesons.
Mesons
The binding among the quarks is so powerful in the baryons, that if sufficient energy is added, these bonds wont break but a new quark plus anti quark pair is created.
This quark + anti quark pair is called a meson.
There are lots of combinations.
I show two common ones below.
Picture: K+ Meson (Kaon)
Picture: Pi + Meson (Pion)
Note that gluons are the force carriers in mesons as well.
Mesons play an important role and contribute to the strong force that keeps the nucleus together
- They are the “effective mediators of the strong force” between larger composite particles like protons and neutrons within an atomic nucleus.
Color Charge
I recommend you watch this Klonusk video starting at time 3:54/19:12 (All Fundamental Forces and Particles Explained Simply | Elementary particles)
Quarks possess a property called color charge, analogous to electric charge but coming in three “colors”: red, green, and blue (and corresponding anticolors for antiquarks).
So think of colors as a type of charge involved in the sub-particles of neutrons and protons.
- The color charge is the source of the strong nuclear force, the most powerful of the four fundamental forces.
The interaction between quarks is mediated by fundamental particles called gluons.
- Color charges constantly move around among the quarks of baryons (and mesons).
- Gluons facilitate this movement.
- Gluons are unique among force carriers because, unlike photons which are electrically neutral, gluons themselves carry color charge (specifically, a color and an anticolor).
- This allows gluons to interact not only with quarks but also with other gluons, leading to the incredibly strong nature of the strong force.
Individual quarks (and gluons) are never observed in isolation, but are always bound together into color-neutral composite particles.
This of course applies to baryons and mesons (and all the other mostly briefly appearing combinations) in protons, neutrons and the nucleus space they occupy.
The Strong Nuclear Force
“The strong nuclear force is the color charge that involves the exchange of gluons between quarks, binding quarks together and creating the strongest force in the universe.” – excerpted from the Klonusk video (All Fundamental Forces and Particles Explained Simply | Elementary particles).
- The range of the strong force is limited to about 2.5 femtometers (2.5E-15 meters).
- The diameter of a proton is less than 1 femtometer, so the strong force extends beyond the neutron and proton.
- the strong force binds the quarks in each neutron or proton but also
- binds the protons and neutrons of a nucleus.
- Otherwise those positively charged protons in the nucleus would repel and move away from each other.
- Mesons play a role here as well
- The gluon is the strongest force carrier.
- The strong force is 100 times stronger than the electromagnetic force.
- It keeps the protons (and neutrons) together in the nucleus (like Velcro).
- It’s this kind of energy that is released from nuclear bombs.
Prepare To Have Your Mind Blown
Let’s do a material balance on the constituents of a proton.
- We know that protons are made of two up quarks and one down quark
- Adding up their masses we get 2.16 + 2.16 + 4.7 MeV/c2 = 9.02 MeV/c2
- But the total mass of a proton is 938 MeV/c2 !
- The resting mass of the quarks makes up only about 9.02/938 = 1% of the total mass !
- So where is the vast majority of the mass?
- It’s in (it is) the massive energy of the strong nuclear force
Somehow, the energy, invisible to us on the quantum microscopic scale, manifests itself as mass on the macroscope scale.
The field of Quantum Chromo Dynamics (QCD) explains how energy eventually becomes a stable particle with mass.
Ok, even though you should be shaking your head due to both wonderment and confusion, you need to keep it together.
We need to talk about leptons.
Leptons
Electrons are part of the Lepton family.
- Three have charges and three (the neutrinos) are neutral.
- Only the electron is involved in atom formation.
- Leptons exist alone and not in composite form like the quarks.
- Neutrinos are tiny with very little mass.
- Electrons, muons, and tau particles are charged and therefore produce an electric field.
- protons (charged composite quarks) also produce an electric field.
Chart: Three Generations of Leptons Excerpted from Wikipedia
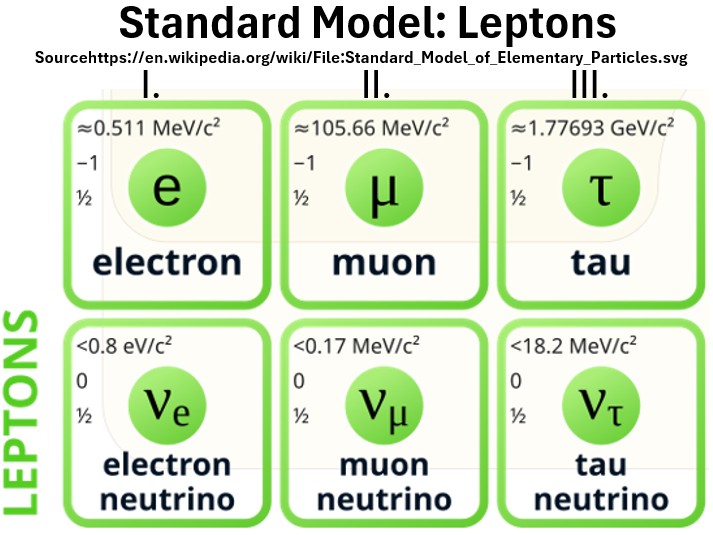
Chart: Leptons
Photons
Photons are the electromagnetic field force carriers.
Particles with charge will attract and repel via the electromagnetic field.
They do this by exchanging photons.
Atoms seek their most stable structure.
- Energized atoms have electrons moving at higher energy levels
- They will tend to seek lower energy levels though.
- When they move from higher to lower energy levels, photons are emitted.
Electromagnetic (EM) Force
- The EM force carrier is the photon.
- Only the strong force is stronger than the gravitational force.
- Electromagnetic forces are 10E36 times the gravitational force.
- But, these forces attract and repel and so will tend to cancel each other.
- On a macroscopic scale (like a planet, star, or even a human body), the vast majority of matter has a net electric charge of zero.
- i.e. in the universe you don’t see large collections of charged forces like you do mass concentrations (and therefore gravitational forces).
- EM forces comprise the Electrostatic (Coulomb Force) and the Magnetic Force.
- Electricity and Magnetism are fundamentally interconnected phenomena, where a changing electric field creates a magnetic field and a changing magnetic field creates an electric field.
- EM waves are generated by accelerating charges.
- EM waves are perpetually produced as each change in one produces the other (and they can travel in a vacuum).
- Electromagnetic (electrostatic and magnetic) forces extend infinitely far away (like gravity).
Coulomb’s Law
- Electrostatic Force (Coulombs Law)
- Fe = kq1q2/r2
- Fe = electrostatic force
- q1, q2 =The quantity of charge of objects 1 and 2
- r = distance between the charges.
- Magnetic Forces also generally follow an inverse square law.
EM forces are responsible for the nature of light.
EM forces are the basis of all chemistry.
Atoms exist due to EM forces binding electrons to their nuclei.
Recap – Strong Force and EM Force
Ok, so far we’ve established that the strong force keeps the nucleus together and is the strongest of all fundamental forces.
- It acts over a very small distance and
- its force carrier is the gluon.
We also know that if particles are charged, they will be affected by the electromagnetic force.
- It is overwhelmed by the strong force at the small ranges of the nucleus,
- but it extends infinitely into space and
- will govern the attraction and repulsion of particles like protons and electrons.
- So, it will keep electrons in orbit (in orbitals actually) around the positive nucleus.
- The force carrier is the photon.
So, we see that the strong force and the electromagnetic force are crucial in maintaining the form and stability of atoms.
Picture: Photons and Gluons Allow Atoms to Exist (Using Helium as an example)
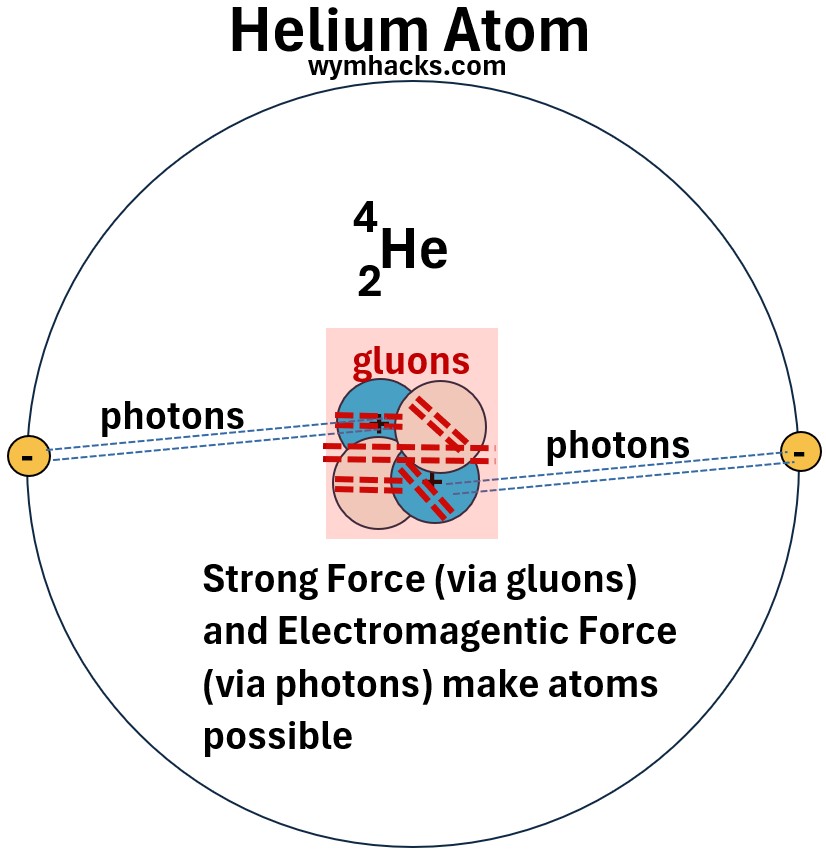
Let’s discuss the weak force now.
The Weak Force
I used Google Gemini in this section.
The weak force
- is responsible for radioactive decay (specifically beta decay) and nuclear fusion processes that power stars, like the Sun.
- is the only fundamental force that changes particle identities (i.e. allows one type of quark or lepton to change into another).
- can transform a proton into a neutron, or vice versa, by changing a quark’s identity.
- is mediated by relatively massive W and Z bosons which
- cause the weak force to have a very short range, much shorter than the electromagnetic or strong forces.
Why is it called the Weak Force?
The weak force is very important and enables radioactive decay.
The sun’s reactions that give us heat and light are enabled by radioactive decay.
It’s called “weak: because of its
- Relative Strength
- When comparing the three forces (strong, electromagnetic, and weak) at the very short distances where they operate, the weak force is the “weakest.”
- Range
- The weak force has an incredibly short range, far smaller than the diameter of a proton.
- The strong force, while also short-ranged, extends further than the weak force.
- Range of the Weak Force: Approximately 10E−18 m (.001 femtometers)
- Diameter of a Proton: Approximately 10E−15 meter (or 1 femtometer).
- Effectiveness in Binding
- The strong force “binds” quarks together to form protons and neutrons, and also binds protons and neutrons together to form atomic nuclei.
- The electromagnetic force binds electrons to nuclei to form atoms, and atoms together to form molecules.
- The weak force doesn’t primarily bind things together. Instead, it’s responsible for transforming particles.
- These transformations often happen much more slowly (compared to the actions of the other forces)
While the weak force is still much stronger than the gravitational force, its “weakness” is relative to the other forces that operate within the nucleus.
Unstable Atoms
Atoms tend to be stable when the ratio of their neutrons to protons (N/P) is in a certain range.
- For smaller atoms the ratio N/P is = 1.
- For heavier atoms (atomic number bigger than about 20), the ratio increase.
- In the heaviest atoms the stable ratio might be more like 1.5: 1.
Any deviation from this “band of stability,”
- whether it’s an excess of neutrons or protons,
leads to an unstable (radioactive) atom that will decay to achieve a more stable configuration.
The weak force enables this tendency towards atomic stability.
Before we look at radioactive decay, lets make sure we understand some atomic nomenclature.
Atomic Number and Mass Number
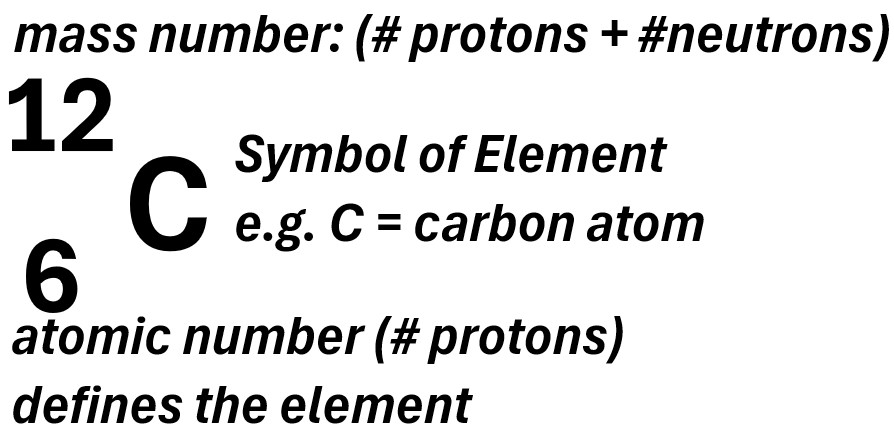
Often you will see atoms being described with the nomenclature shown in the carbon atom example below.
Picture: Atomic Nomenclature Example (Carbon)
- The middle letter (or letters sometimes) denotes the atomic symbol e.g. C = carbon
- The lower number is called the atomic number
- The atomic number is the number of protons in the atom.
- Each atom has a unique atomic number.
- For neutral atoms (non ionic), the number of electrons will equal the number of protons
- The upper number is called the mass number
- The mass number = the number of protons plus the number of neutrons
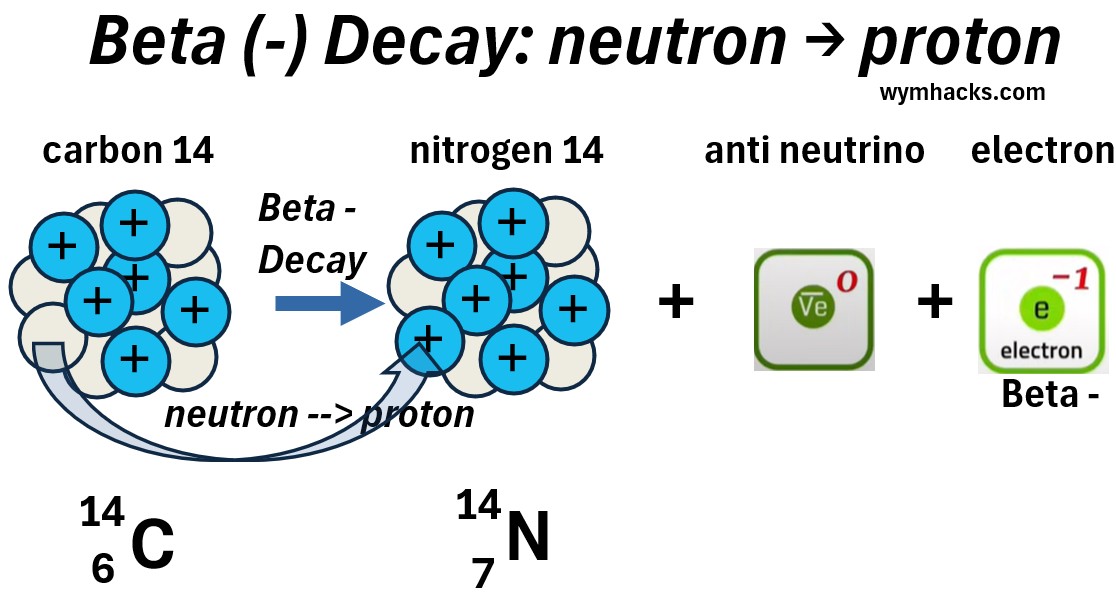
Beta – (Beta Minus) Decay
When the neutron of an atom transforms into a proton, we have Beta Minus (-) Decay.
Beta Minus (or Beta -) just refers to production of an electron (a Beta – particle).
In Beta minus decay, an anti neutrino is also produced.
Picture: Carbon 14 Beta Minus Decay to Nitrogen 14
In the example reaction above, a carbon 14 atom
- decays into a nitrogen 14 atom
- plus an anti neutrino and an electron.
Let’s drill down to a single neutron to proton transition, to see what reactions are occurring.
Picture: Standard Particle Perspective of a Carbon 14 Beta Minus Decay to Nitrogen 14
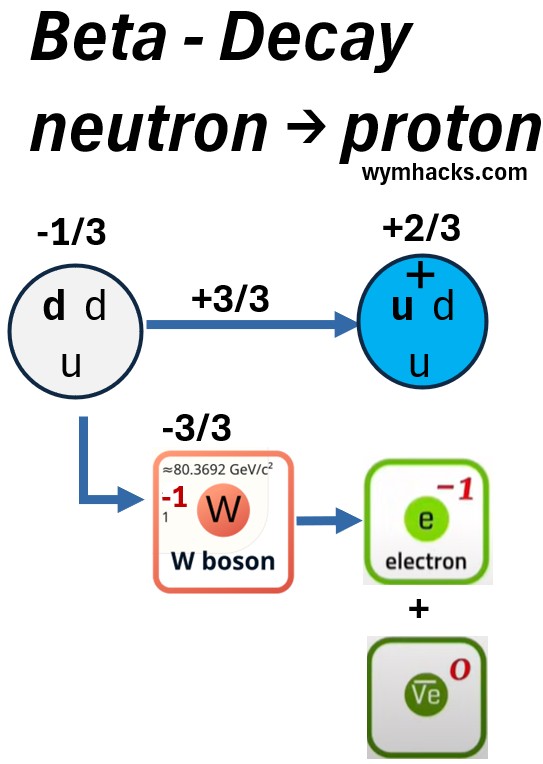
In Beta minus decay,
- one of the down quarks in a neutron, changes to an up quark
- The ddu quark configuration (neutron) is now a udu configuration (proton).
- The charge goes from -1/3 to 2/3
- A -1 W boson is created to effectively balance the charge.
- The W boson quickly decays into an electron (a lepton) and an anti neutrino (an anti lepton)
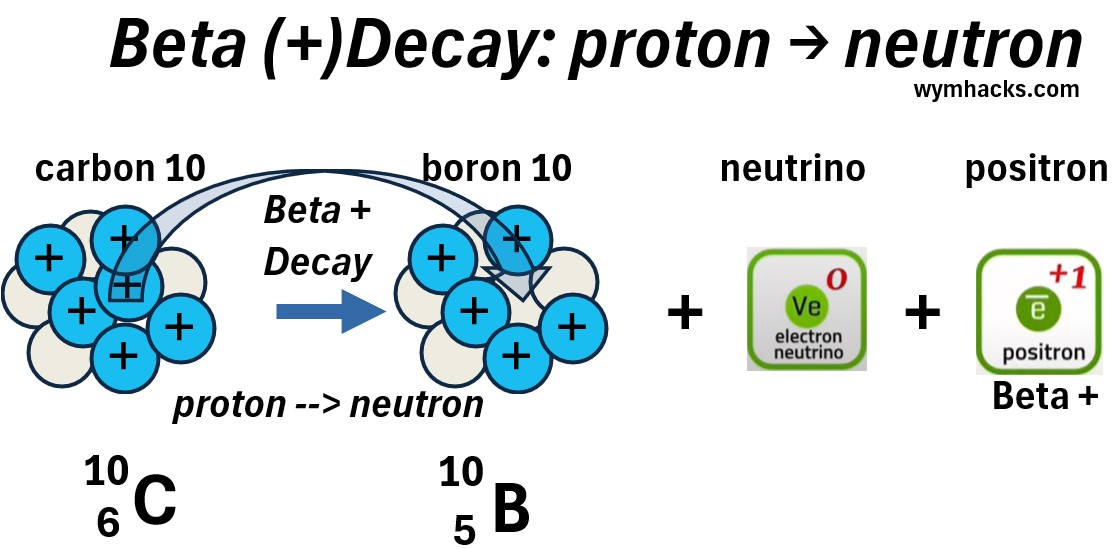
Beta + (Beta Plus) Decay
When the proton of an atom transforms into a neutron , we have Beta Plus Decay.
- As a memory trigger, associate the Plus in Beta Plus with Proton conversion.
Beta Plus (or Beta +) just refers to the production of a positron (a Beta + particle).
In Beta plus decay, a neutrino is also produced.
Picture: Carbon 10 Beta Plus Decay to Boron 10
In the example reaction above, a carbon 10 atom decays into a boron 10 atom plus a positron and a neutrino.
Let’s drill down to a single proton to neutron change, to see what reactions are occurring.
Picture: Standard Particle Perspective of a Carbon 10 Beta Plus Decay to Boron 10.
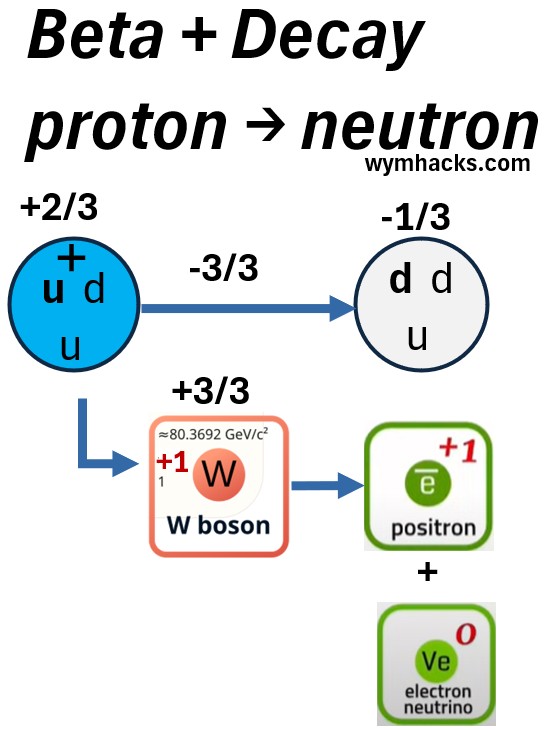
In Beta plus decay,
- one of the up quarks in a proton, changes to a down quark.
- The udu quark configuration (proton) is now a ddu configuration (neutron)
- The charge goes from +2/3 to -1/3
- A +1 W boson is created to effectively balance the charge.
- The W boson quickly decays into a neutrino (a lepton) and a positron (an anti lepton).
Weak Interaction Beta Decays are Crucial to Life
I used Google Gemini to provide some of the descriptions given below.
The weak force is crucial for our existence and the universe as we know it.
- Nuclear Fusion
- All stars ,including the sun, generate energy through nuclear fusion.
- In the the proton-proton chain reaction that powers the Sun, a proton beta plus decays to a neutron to form deuterium.
- Deuterium is necessary for the subsequent fusion reactions that create helium and release enormous amounts of energy.
- No weak force means no light and no warmth! (see Byron’s version of this).
Nuclear fusion involves beta plus decay as shown in the picture below.
Picture: Solar Nuclear Fusion (Source: Wiki)
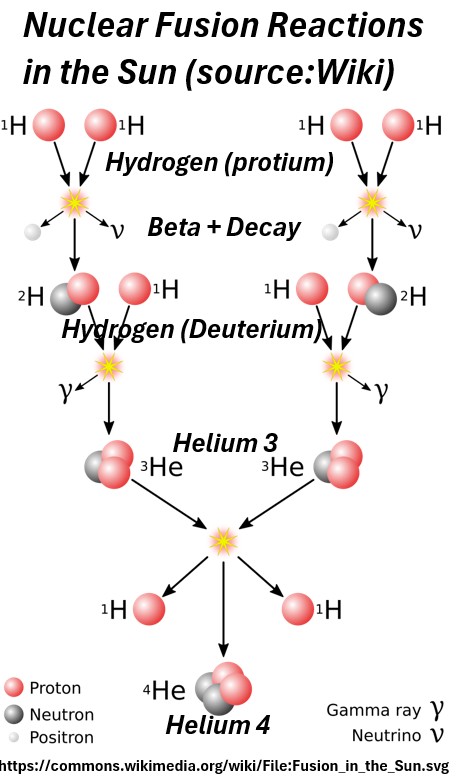
- The weak force creates elements (Stellar Nucleosynthesis):
- Beyond just hydrogen and helium, the weak force plays a key role in the creation of heavier elements inside stars.
- The ability of the weak force to change the “flavor” of quarks (e.g., turning protons into neutrons and vice versa) is essential for the processes that build up heavier nuclei.
- All the carbon in our bodies, the oxygen we breathe, the silicon in the ground, and virtually all other elements heavier than helium were forged in stars, and the weak force was a necessary ingredient in their formation.
- When these stars explode as supernovae, they scatter these elements throughout the universe, providing the building blocks for planets and life.
- The weak force is the underlying mechanism in various technologies like Radiometric Dating and Medical Imaging and Treatment.
- Geothermal Heat
- The Earth’s internal heat,
- which drives geological processes like plate tectonics and volcanism,
- is partly generated by the radioactive decay of elements within the Earth’s core, a process facilitated by the weak force.
- Neutrinos and Understanding the Universe
- The weak force is the only force that neutrinos (tiny, elusive particles) interact with, other than gravity.
- Studying neutrinos produced by the Sun or in nuclear reactors helps physicists understand not only the Sun’s interior but also the fundamental properties of neutrinos themselves, which are important for our understanding of the universe.
Nuclear Fission Involves Beta Decay
Nuclear fission is
- a nuclear reaction in which
- the nucleus of a heavy atom splits into two or more smaller, lighter nuclei.
This process is accompanied by
- the release of a tremendous amount of energy,
- along with other particles, typically neutrons and gamma rays.
Nuclear fission generally happens with very heavy, unstable elements like Uranium and Plutonium.
The smaller nuclei formed during fission
- are often neutron-rich and therefore radioactive,
- undergoing further radioactive decay (like beta-minus decay) until they reach a stable state.
The +- W bosons are the force carriers of the weak force in the process of Beta decay.
What about the Z bosons?
The Z Boson
Z bosons are fundamental force-carrying particles that mediate the weak nuclear force.
- Unlike W bosons, they are electrically neutral, but
- like W bosons, they are very massive, which gives the weak force its extremely short range.
- They appear when particle /anti particle pairs collide.
Particle + Anti Particle → Z boson → Another Particle + Another Anti Particle
Picture: Z Boson Creation from a Quark / Anti Quark Pair
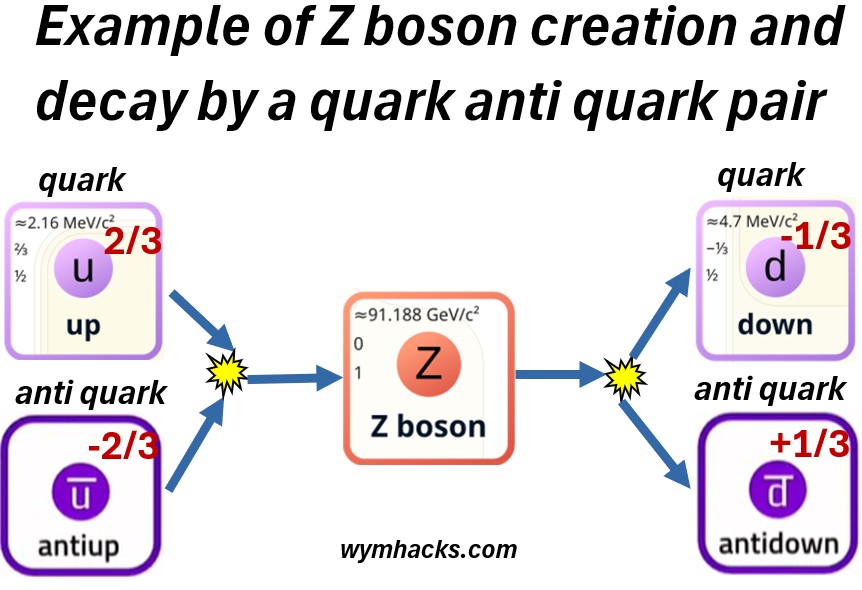
Picture: Z Boson Creation from a Lepton / Anti Lepton Pair
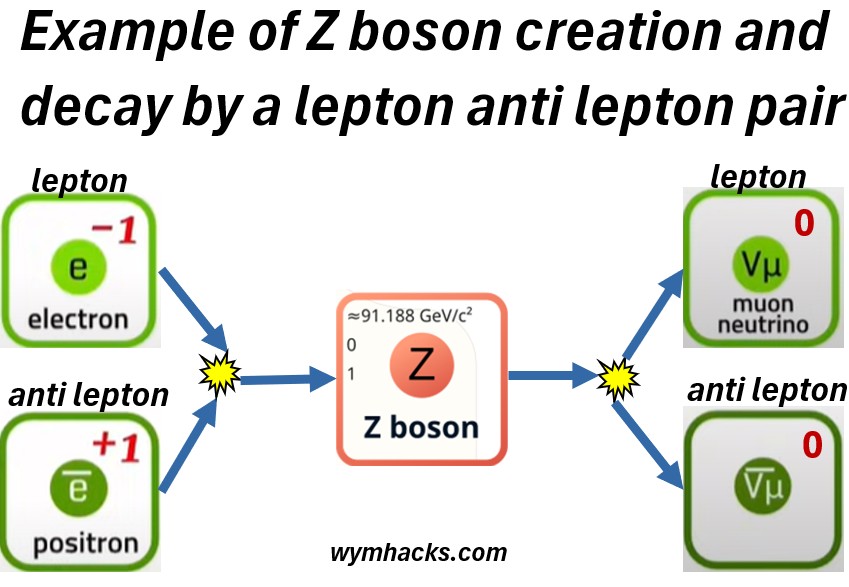
Z bosons
- are crucial for a complete understanding of the weak force and
- played a key role in confirming the Electroweak Theory (see next section).
Their discovery at CERN in 1983, along with W bosons, provided strong experimental evidence for the unification of the electromagnetic and weak forces into a single electroweak force, a cornerstone of the Standard Model of particle physics.
Electroweak Theory
The Electroweak Theory unifies two of the four fundamental forces of nature:
- the electromagnetic force and
- the weak nuclear force.
The Electroweak Theory says that,
- deep down, the electromagnetic and weak forces aren’t actually separate.
- They’re like two sides of the same coin.
- When the universe was super young and incredibly hot, these two forces were actually one unified “Electroweak Force.”
- As the universe cooled down, a special field called the Higgs Field “froze” into place throughout space.
- This Higgs Field is like a cosmic sticky syrup.
- It affected the particles that carry the weak force (the W and Z bosons),
- making them heavy and slow,
- which is why the weak force only works over tiny distances and seems “weak.”
- But the particle that carries the electromagnetic force (the photon) didn’t get stuck in the syrup, so it remained massless and can travel forever,
- which is why the electromagnetic force has such a long reach.
- So, the Electroweak Theory tells us that these two seemingly different forces are actually just different ways we see one underlying force, depending on the energy conditions of the universe.
W and Z Bosons Decay Quickly
Many of the standard particles appear and then disappear quickly.
Check out some of the lifetimes in the picture below.
Picture : Standard Model Elementary Particle Lifetimes
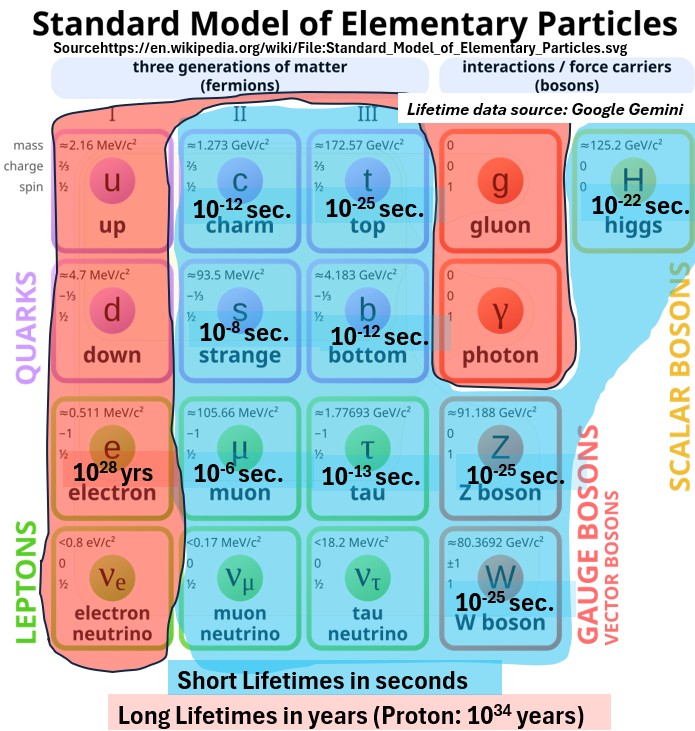
- A proton, made of up and down quarks lasts a very long time (10E34 years!) while
- Z and W bosons last an infinitesimal 10E-25 seconds!
Only first generation particles (Gen I) make up most of the matter in the universe.
So where did we discover these things?
Many Standard Model particles were discovered at major particle accelerator laboratories around the world.
Key locations include:
- CERN (European Organization for Nuclear Research) in Switzerland:
- Responsible for the discovery of the W and Z bosons (1983) and the Higgs boson (2012).
- It also contributed to early evidence for quarks and continues to be a hub for particle physics.
- SLAC (Stanford Linear Accelerator Center) in the USA:
- Provided the first experimental evidence for quarks (specifically the up, down, and strange quarks) in the late 1960s.
- The tau lepton was also discovered there in 1976.
- Fermilab (Fermi National Accelerator Laboratory) in the USA:
- Discovered the bottom quark in 1977 and the top quark in 1995.
- Neutrino research, including the direct observation of the tau neutrino, has also taken place here
Higgs Boson and Higgs Field
I used Google Gemini to help me with the text in this section.
Imagine the entire universe is filled with an invisible, omnipresent “syrup” called the Higgs field.
- The Higgs boson is simply a tiny ripple or vibration in that syrup. (think of a wave, the Higgs boson, in a lake, the Higgs Field).

The picture above is a snippet from a you tube video (The “God Particle”: a story of Big Science, Big Data, and Human Ingenuity): You can go to time 18:52/58:27 to see the lecturer describe this analogy.
- As fundamental particles (like electrons and quarks) move through this Higgs field, they interact with it,
- and this interaction is what gives them their mass. (think of sail boats on a lake)
- The more a particle “drags” or interacts with the Higgs field, the more massive it becomes.
- Particles like photons (light particles) don’t interact with the Higgs field at all,
- which is why they (photons) have no mass and always travel at the speed of light.
Higgs Postulation and Discovery
The Higgs boson was postulated in 1964 by several physicists, most notably Peter Higgs, François Englert, and Robert Brout.
It was discovered on July 4, 2012, by the ATLAS and CMS collaborations at the Large Hadron Collider (LHC) at CERN in Switzerland.
Higgs Relationship to the four fundamental forces
The Higgs Boson and Higgs Field relate to the four fundamental forces in the following ways:
They relate by:
- Giving Mass to Force Carriers (weak force):
- The Higgs field gives mass to the W and Z bosons (weak force carriers).
- Without the Higgs field, these particles would be massless,
- and the weak force would be long-ranged, like the electromagnetic force.
- The fact that the W and Z bosons are massive is why the weak force only acts over incredibly short distances (within the nucleus).
- This mechanism is a central part of the Electroweak Theory, which unifies the electromagnetic and weak forces.
- No Direct Effect on Photons (electromagnetic force)
- The Higgs Field does not give mass to the photon, the carrier of the electromagnetic force.
- This is why the electromagnetic force has an infinite range, as its mediator is massless.
- No Direct Role in Strong Force or Gravity (as far as we know):
- The Standard Model currently says the Higgs field does not directly give mass to the gluons (carriers of the strong force), which are massless.
- Similarly, it’s not involved in giving mass to hypothetical gravitons (carriers of gravity), if they exist.
- The strong force’s strength and range are explained by its own unique properties, and
- gravity remains outside the Standard Model’s full description.
In essence, the Higgs field
- acts like a cosmic “mass-giver” to certain fundamental particles, and
- its interaction with the W and Z bosons is what makes the weak force distinct from the electromagnetic force at lower energies.
The Gravitational Force
I used Google Gemini to help me write parts of this section.
Gravitational force governs the attraction between any two objects that have mass or energy.
It’s what keeps us on the ground, holds planets in orbit around stars, and forms the large-scale structures of the universe like galaxies.
Isaac Newton’s Law of Universal Gravitation (published in 1687) describes gravity as a force of attraction between any two objects with mass.
Newton’s law states that the strength of this force depends on two main things:
- The masses of the objects: The more massive the objects, the stronger the gravitational pull between them.
- The distance between them: The farther apart the objects are, the weaker the gravitational pull.
Gravitational Force can be expressed in equation form as:
- Fg = G(m1)(m2)/r2
- Fg = gravitational force
- G= Universal Gravitational Constant
- m1, m2 = mass of objects 1 and 2
- r = distance between objects
Newton’s laws of gravity works very accurately for most practical problems and everyday situations.
Theory of Relativity
Albert Einstein’s General Theory of Relativity, offers a deeper understanding of gravity as a curvature of spacetime itself, rather than just a simple force.
According to Einstein:
- Gravity is not a push or pull force in the traditional sense.
- It’s a manifestation of the curvature of spacetime caused by the presence of mass and energy.
- Massive objects warp the fabric of spacetime around them, and other objects then follow these curves, giving the appearance of being pulled by a force.
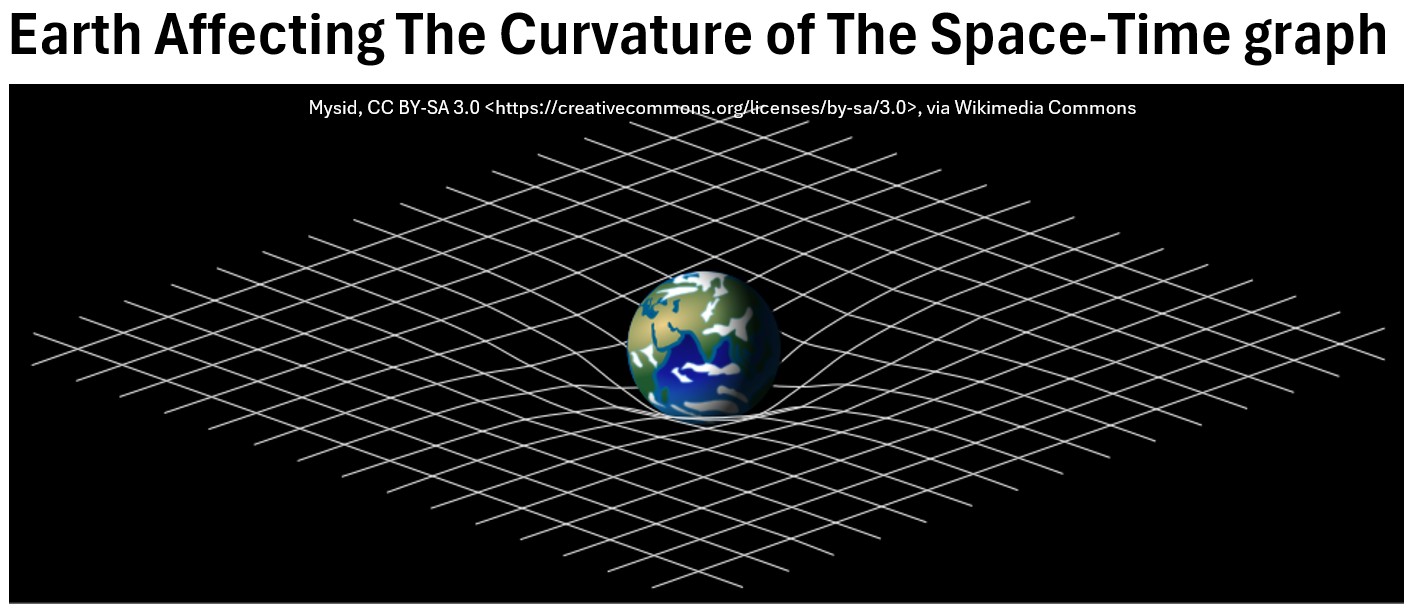
Despite its immense influence across the universe, gravity is the weakest of the four fundamental forces in nature.
Gravitational Force Is Not Part of the Standard Model
Gravitational force is not part of the Standard Model of particle physics.
- This is because the Standard Model successfully describes the other three fundamental forces—the strong, weak, and electromagnetic forces—using quantum field theory.
- But gravity, as described by Einstein’s General Relativity, operates on a different mathematical framework (spacetime curvature) that has proven notoriously difficult to reconcile with quantum mechanics.
- While physicists theorize a quantum carrier for gravity called the graviton, it has not been observed.
- Attempts to incorporate gravity into the Standard Model’s quantum framework lead to mathematical inconsistencies,
- highlighting the need for a more comprehensive “theory of everything” that can unify all four fundamental forces.
Summary of Gravity
- Gravity is not included in the Standard Model.
- According to theory, its force carrier is the graviton but this has not been proven.
- It’s the weakest of the four fundamental forces.
- Gravity keeps you “glued” to earth and keeps the planets in orbit.
- It is an attractive force.
For most practical phenomena, Newton’s Laws of Gravitation describes its behavior accurately.
- Fg = G(m1)(m2)/r2
- Fg = gravitational force
- G= Universal Gravitational Constant
- m1, m2 = mass of objects 1 and 2
- r = distance between objects
- Gravitational Force extends infinitely far away.
Summary
My company once sent me to a training class on how to make effective presentations, which basically boiled down to the following rules:
- Tell them what you are going to tell them (the introduction),
- then tell it to them (the details),
- then tell them what you told them (summary of the details).
So, now I’m going to tell you what I told you.
Standard Model
The Standard Model describes
- the fundamental particles (quarks, leptons, and their antimatter counterparts) and
- three of the four fundamental forces (strong, weak, and electromagnetic) that govern how they interact,
- all mediated by force-carrying particles (bosons) and with the Higgs boson providing mass.
- It notably excludes gravity.
Building Blocks of Matter
- All matter is made of elementary particles that are one of two types: quarks or leptons.
- Quarks and leptons each consist of six types of particles.
- The six types are related in pairs, or “generations”.


Forces
There are four fundamental forces at work in the universe:
- the strong force,
- the electromagnetic force,
- the weak force, and
- the gravitational force.
They work over different ranges and have different strengths.
- Gravity and electromagnetic force have infinite range.
- The weak and strong forces are effective at subatomic levels .
- The forces from weakest to strongest are: gravity, the weak force, the electromagnetic force, and the strong force.
Force Carriers
- Three of the fundamental forces result from the exchange of force-carrier particles, which belong to a broader group called “bosons”.
- Particles of matter transfer discrete amounts of energy by exchanging bosons with each other.
- Each fundamental force has its own corresponding boson –
- The Standard Model includes the electromagnetic, strong and weak forces and all their carrier particles, and explains well how these forces act on all of the matter particles.
- However, the most familiar force, gravity, is not part of the Standard Model.
Physicists believe that all these forces come from one underlying force.
Picture: The Force Carriers (Gluons, Photons, W and Z Bosons)
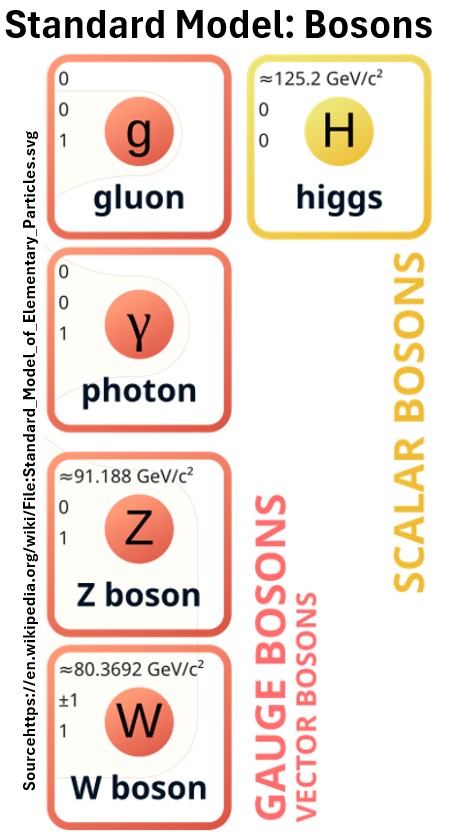
The Strong Force
- The strongest force carrier is the gluon.
- The strong force is 100 times stronger than the Electromagnetic Force.
- It keeps the protons (and neutrons) together in the nucleus (like Velcro).
Picture: A Proton: The Strong Force Carrier Gluons Keep Quark Particles together
- The Strong Force is only effective at small scales (the width of a proton).
- It’s this kind of energy that is released from nuclear bombs.
Electromagnetic (EM) Force
- The EM force carrier is the photon.
- Electromagnetic Forces are 1036 times the Gravitational Force.
- They are 10 to 12 times stronger than the Weak Force.
- But, these forces attract and repel and so will tend to cancel each other.
- On a macroscopic scale (like a planet, star, or even a human body), the vast majority of matter has a net electric charge of zero.
- i.e. in the universe you don’t see large collections of charged forces like you do mass concentrations (and therefore gravitational forces)
- EM forces comprise the Electrostatic (Coulomb Force) and the Magnetic Force.
- Electricity and Magnetism are fundamentally interconnected phenomena, where a changing electric field creates a magnetic field and a changing magnetic field creates an electric field.
- EM waves are generated by accelerating charges.
- EM waves are perpetually produced as each change in one produces the other (and they can travel in a vacuum).
- Electromagnetic (electrostatic and magnetic) forces extend infinitely far away (like gravity).
- Electrostatic force (Coulomb’s law)
- Fe = kq1q2/r2
- Fe = electrostatic force
- q1, q2 =The quantity of charge of objects 1 and 2
- r = distance between the charges.
- Magnetic forces also generally follow an inverse square law.
- EM forces are responsible for the nature of light.
- EM forces allow atoms to exist , keeping electrons bound to nuclei.
Picture: Gluons and Photons Allow Atoms to Exist (Example Helium Atom)

- EM Forces are the basis of all chemistry.
The Weak Force (The Weak Interaction)
- The weak force carriers are the W and Z bosons.
- The weak force is 1025 times the strength of gravity.
- It’s only effective at really small scales (1/1000 the diameter of a proton).
- Responsible for radioactive decay. (beta minus or beta plus decay)
- Example: Cesium decay
- Cs → Ba + electron + anti-electron neutrino
- Cesium 137 has 137 nucleons (protons + neutrons)
- It’s Cesium because it has exactly 55 protons.
- One of the Neutrons (a quark flips) turns into a proton
- The element now has one extra proton (56 total) and is now Barium.
- An electron and an anti-electron neutrino are produced.
- Beta plus decay gives us energy and light from the sun
Picture: Beta Plus Decay (Fusion in the Sun involves Beta Plus Decay)
Gravitational Force
- Gravity is not included in the Standard Model.
- It’s theorized that its force carrier is the graviton but this has not been proven.
- It’s the weakest of the forces.
- Gravity keeps you “glued” to earth and keeps the planets in orbit.
- It is an attractive force.
- For all practical purposes Newton’s Law of Gravitation describes gravity
- Fg = G(m1)(m2)/r2
- Fg = gravitational force
- G= Universal Gravitational Constant
- m1, m2 = mass of objects 1 and 2
- r = distance between objects
- Gravitational Force extends infinitely far away.
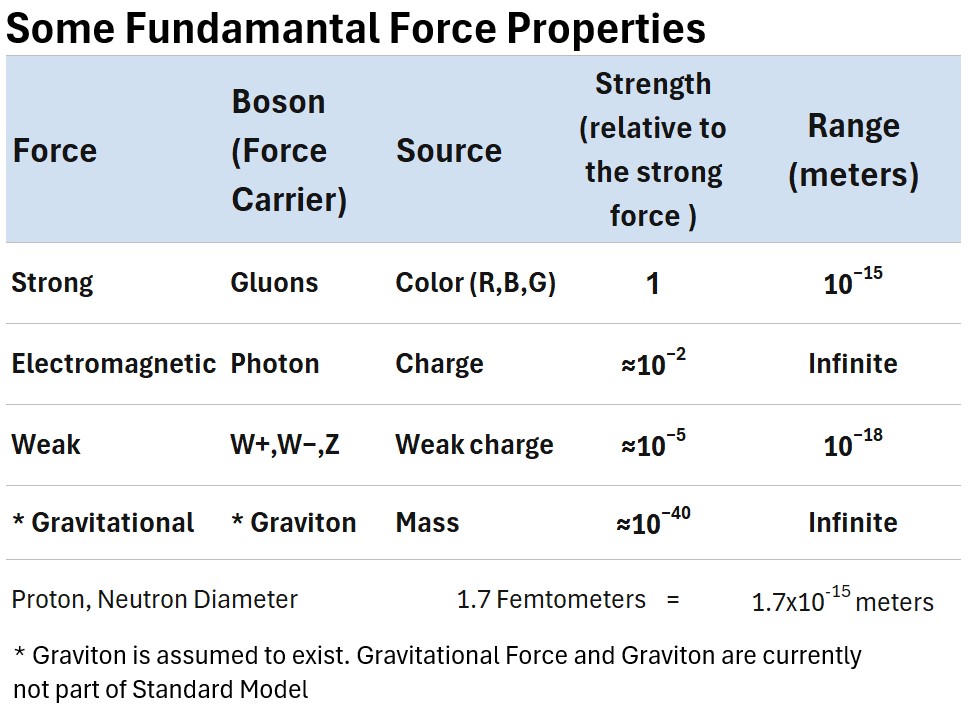
The table below summarizes the relative strengths and ranges of the four fundamental forces.
Table: Comparing Forces and Force Carriers
I annotated the Wiki Standard Model chart below to show the range in which the various force carriers operate.
Picture: Impact of Bosons on Various Standard Particles
Namely:
- Gluons operate at close distance and keep quarks together (and therefore protons and neutrons and nuclei together).
- The range of photons and W, Z Bosons (and the theoretical gravitons) are much wider
- The Higgs Field gives particles their mass
Conclusion
We’ve covered a lot of ground in this discussion, some of which is quite complex.
The Standard Model of particle physics effectively explains many of the universe’s fundamental forces and their interactions.
However, it’s not a complete picture because gravity, the force we experience most directly, isn’t incorporated within it.
I’ve briefly mentioned that gravity is described by a separate framework, Einstein’s Theory of Relativity, which uses a different mathematical approach.
Scientists hope to integrate gravity into the Standard Model, ultimately creating a unified “theory of everything.”
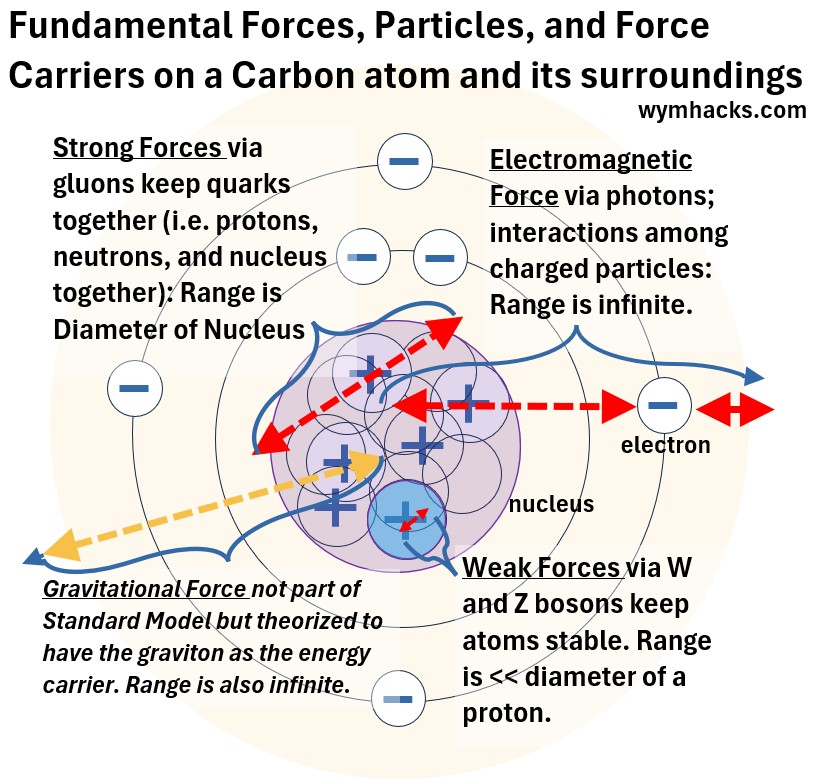
Picture: Fundamental Forces and Force Carriers on a Carbon Atom and its Surroundings
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.