Menu
SI Base Units
Last Revision: July 13, 2025
Introduction
This post describes the 7 SI Base Units.
I first give you a basic primer on scientific notation because some of the base units deal with very big and very small numbers.
Then, I provide some historical and definitional background (Base vs Derived Units for example) as well as describe the universal constants that are used with the Base Units.
We then finish up with more detailed definitions for each of the 7 Base Units.
My main source for this information is the nist.gov website (SI Units) which provides in depth information and fantastic graphics.
Exponentiation and Scientific Notation
Exponentiation
- xy = x raised to the y power; e.g. r2 = r squared= rr = r*r
- x-y = one over x raised to the y power = 1/xy
- e.g. r-2 = 1/r2
- e.g. a-3b2 = (1/a3)b2
“Times 10 to the power of” Notation
Scientific notation
- Known as the standard form,
- “x 10y” means times 10 to the power y
- e.g. 245,000 = 2.45 x 105
- e.g. 23,400,000 = 23.4 x 106
- e.g. .00234 = 23.4 x 10-4
E (or e) notation
- is short hand for “times 10 to the power of”.
- e.g. 245,000 = 2.45e5 = 2.45E5 = 2.45 x 105
- e.g. 1e6 = 1E6 = 1,000,000 = 1 x 106
- e.g. 1e-3 = 1E-3 = .001 =1 x 10-3
Engineering notation
- is a type of scientific notation where the exponent is always a multiple of 3.
- e.g. 245,000 = 245E3 = 245 x 103
See wikipedia.org for more on scientific notation.
SI Base Units
SI (International System of Units) defines seven base units which are
- meter
- kilogram
- second
- ampere
- kelvin
- mole
- candela
The definition of the mole, alongside redefinitions of the kilogram, ampere, and kelvin (and candela)
- were formally approved by the General Conference on Weights and Measures (CGPM)
- on November 16, 2018, and
- officially took effect on May 20, 2019 (World Metrology Day).
The base units form the foundation upon which all other units of measurement are built.
- Base Units are Independent Physical Quantities:
- They represent a distinct and independent physical quantity.
- They are irreducible dimensions of physical phenomena.
All other units in the SI system are called derived units which are combinations of base units.
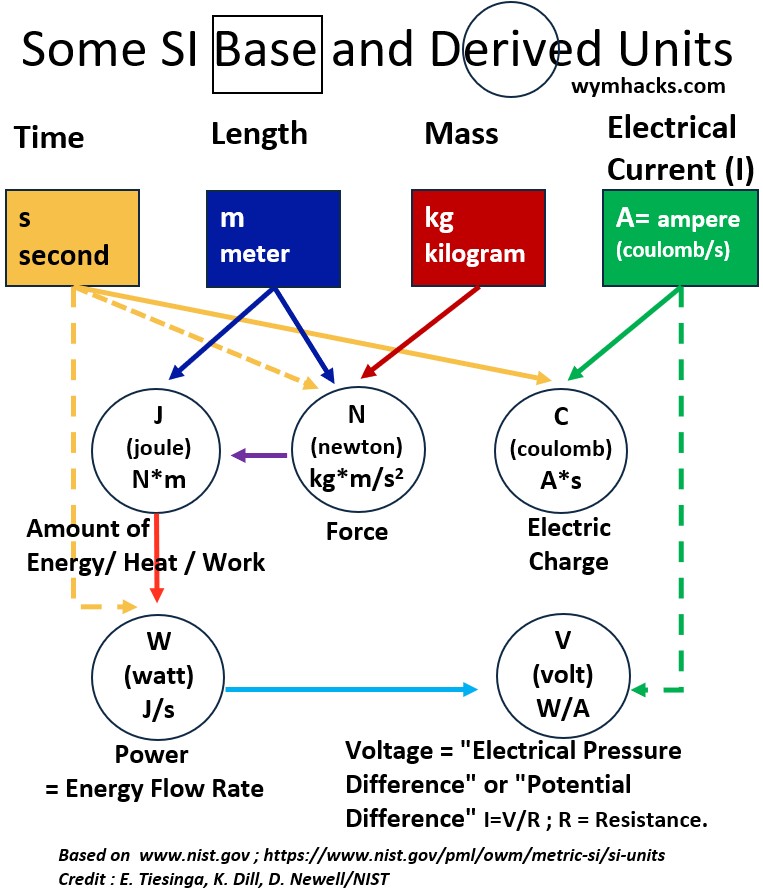
I show the base and derived unit relationship in the schematic below for four of these units (time, length, mass, and current).
Picture – Some SI Base and Derived Units
The derived units shown in the picture above are
- Energy: 1 joule = 1 newton-meter = 1 Nm
- Force: 1 newton = 1(kilogram-meter)/(second squared) = 1 (kgm)/(s2)
- Charge: 1 coulomb = 1 ampere-second = 1As
- Power: 1 watt = 1 joule/second = 1 J/s
- Voltage: 1 volt = 1 watt/ampere = 1 W/A
The SI units all are based on known constants and they are the same everywhere in the universe.
These constants are:
- c: the speed of light in vacuum: 299,792,458 meters per second.
- h: the Planck constant: Energy is exchanged and absorbed in specific amounts, known as “quanta”
- and h defines the size of those quanta.
- h equals exactly 6.626 070 15 × 10-34 Joule seconds.
- e: elementary charge: e is the amount of charge in an electron
- and is equal to 1.602176634 x 10-19 coulombs.
- ∆νCs: the hyperfine transition frequency of cesium-133 = 9,192,631,770 hertz.
- k: the Boltzmann constant relates an object’s energy to its temperature.
- k equals 1.380649 x 10-23 joules/kelvin.
- NA: the Avogadro constant defines the number of particles in a mole
- and equals 6.02214076 x 1023 particles per mole.
- Kcd: the luminous efficacy of monochromatic radiation of frequency 540 × 1012 hertz.
- Kcd is equal to 683 lumens per watt
Specific definitions for the SI base units are provided below.
SI Base Unit Definitions
Time: second = s
(adopted in 1967): The second is defined as the
- duration of exactly 9,192,631,770 cycles of the microwave radiation
- corresponding to the transition between the two hyperfine levels of the ground state of the caesium-133 atom.
Length: meter = m
- The meter is based on the definition of the speed of light c = 299,792,458 meters per second (or m/s)
- (adopted in 1983): 1 meter is the length of the path traveled by light
- in vacuum
- during a time interval of 1/299,792,458 of a second.
Mass: kilogram = kg
(adopted 2019): The kg is defined by taking
- the fixed numerical value of the Planck constant h to be 6.626 070 15 x 10–34
- when expressed in the unit J s, which is equal to kg m2 s–1
Current: ampere = A
- Its magnitude is set by fixing the numerical value of the elementary charge to be equal to exactly 1.602176634 x 10-19 when
- it is expressed in the SI units As [ampere seconds],
- which is equal to C (Coulombs).
- (adopted 2019): Per NIST, “The ampere is a measure of the amount of electric charge in motion per unit time ― that is, electric current.
- But the quantity of electric charge by itself, whether in motion or not, is expressed by another SI unit, the coulomb (C).
- One coulomb is equal to about 6.241 x 1018 electric charges (e).
- One ampere is the current in which one coulomb of charge travels across a given point in 1 second.”
Temperature: kelvin = K
- (Adopted 2019): Its magnitude is set by fixing the numerical value of the Boltzmann constant to be
- equal to exactly 1.380649 x 10-23 … J/K [joules per kelvin].
- The Boltzmann constant relates an object’s energy to its temperature.
- So a Kelvin is the change of thermodynamic temperature
- that results in a change of thermal energy by 1.380649x 10-23 … Joules.
- t (in degrees Celsius) = T (in kelvin) – 273.15
- Absolute zero on the kelvin scale (0 K) is -273.15 degrees Celsius (-273.15 0C )
- TR (Rankine = R) = 1.8 TK (kelvin = K)
- t (in degrees Fahrenheit) = T (in Rankine) – 459.67
- t (0F) = 1.8t(0C ) + 32
Mole: mol
- (Adopted 2019): 1 mole contains exactly 6.02214076 x 1023 elementary particles.
- This number is the fixed numerical value of the Avogadro constant (NA),
- when expressed in the unit mol⁻¹ (reciprocal mole), and
- is called the Avogadro number.
- The “elementary entities” can be
- atoms, molecules, ions, electrons, any other particle, or
- specified groups of such particles.
Luminous Intensity: Candela: cd
- A candela is the SI unit of luminous intensity.
- (adopted 2019): Defined by taking
- the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency 540 x 1012 Hz, Kcd ,
- to be exactly 683 when expressed in the unit lm/W (lumen per watt),
- which is equal to cd⋅sr⋅/W (candela steradian per watt).
- It refers to light at a very specific frequency (540 x 1012 Hz),
- which corresponds to green-yellow light,
- the color to which the human eye is most sensitive under normal daylight conditions.
- The “luminous efficacy” (Kcd) is the conversion factor
- between the purely physical power of light (measured in watts) and
- its perceived brightness (measured in lumens).
- By fixing Kcd to 683 lm/W for this specific frequency,
- the definition essentially establishes the amount of light,
- as perceived by the average human eye,
- that corresponds to one candela.
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.