Menu (linked Index)
Enzyme Primer
Last Update: October 25, 2024
Introduction
- We’ll explore the concept of Activation Energy and how enzymes reduce it.
- We’ll use Reaction Coordinate Diagrams and Maxwell-Boltzmann distribution curves to visualize this concept.
- Additionally, we will examine a specific biological enzyme and its cofactor to illustrate their collaborative role in catalyzing reactions.
Activation Energy
Many important biological reactions require an energy push or impetus to get started and proceed.
- This “required energy” is called the Activation Energy.
- Whether a reaction will occur or not depends on the collisions among the molecules and
- whether the collisions have sufficient energy and orientation.
Without some assistance to overcome their Activation Energy, many critical reactions in our bodies would not occur or would occur so slowly as to to render them ineffective.
- More than 1,000 different types of proteins in the human body (called Enzymes) serve this purpose by reducing the Activation Energy and thus catalyzing the formation of Products from Reactants.
Reaction Coordinate Diagram
Take a look at the chart below where we have a spontaneous reaction (i.e. Reactants will readily or quickly go to Products given sufficient Activation Energy).
In this example,
- the reaction will only occur if their is sufficient energy (i.e. Ea) to overcome the peak of the curve shown in the diagram.
- The presence of an Enzyme has the effect of reducing this Activation Energy “hump” and
- speeding up the reaction (shown by the red dotted line with a catalyzed Activation Energy Ea,c).
Picture_Reaction Coordinate & Activation Energy
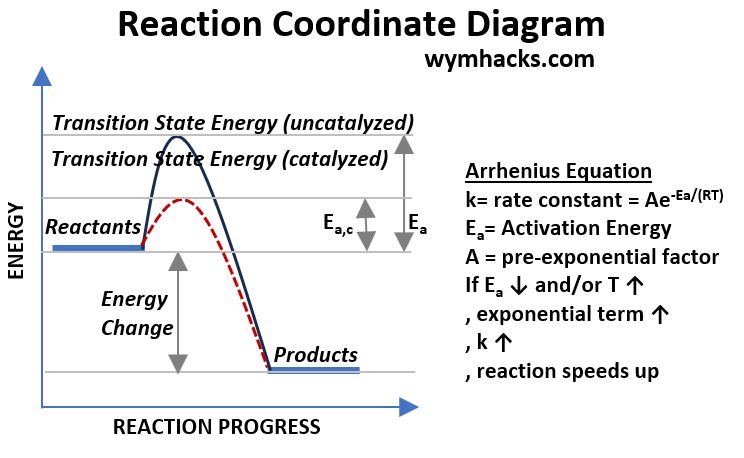
So, even though there is a net release of energy in this example (Energy Products – Energy Reactants = Negative Value), there was still a energy hump or barrier that the reaction had to pass through).
With the Enzyme, the barrier was reduced, resulting in the speeding up of Product formation.
Arrhenius Equation & Maxwell Boltzmann Distribution
According to the Arrhenius Equation [ k = Aexp(-Ea/(RT)) ]:
- the reaction rate (k) will increase if
- the Activation Energy (Ea) is lowered
- and/or if the Temperature (T) is increased.
Maxwell- Boltzmann Distribution curves can help us get a better conceptual understanding of what is happening on a molecular level.
In the chart below,
- the number of molecules involved in a potential reaction are represented by the area under the curve.
- Continuing our hypothetical example from above,
- both the catalyzed and uncatalyzed Activation Energies Ea,c and Ea are plotted as vertical dotted lines.
- The Enzyme has shifted the vertical line from right to left, increasing the area under the curve and
- therefore increasing the number of molecules with sufficient energy to react and form products.
Picture_Maxwell-Boltzmann Distribution for Catalyzed vs Uncatalyzed Reaction
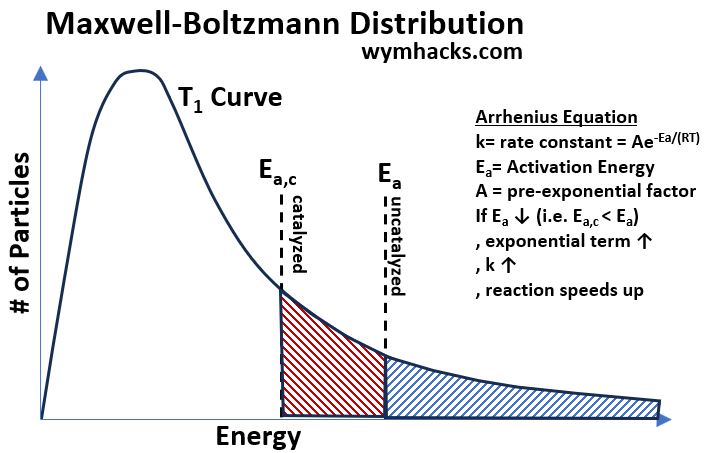
The Arrhenius Equation [ k = Aexp(-Ea/(RT)) ] also tells us that increasing the temperature will speed up the reaction of Reactants to Products.
Maxwell-Boltzmann Distribution lines can show this as well (see the chart below) .
Picture_Maxwell-Boltzmann Distributions at Different Temperatures
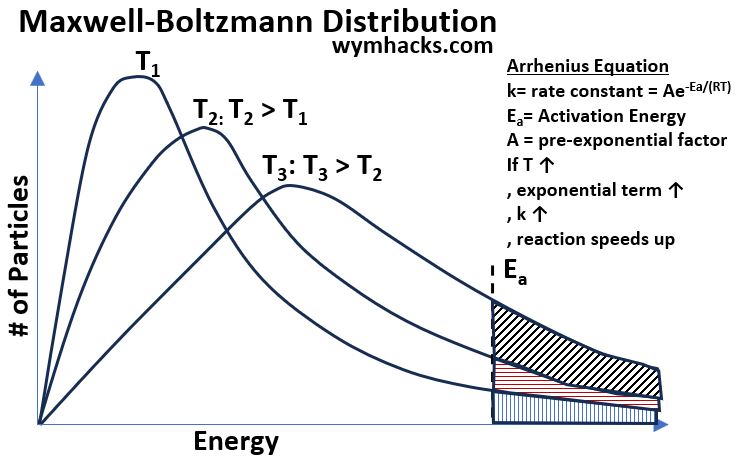
This (temperature effect) is not really relevant for biological systems since temperature is going to be relatively constant,
- but it’s cool to see how these curves give a nice visual display of the Arrhenius Equation in action.
- With an increase in temperature, for the same population of molecules, the curves flatten and spread out more into the high energy region.
- The end result is more “higher energy” molecules available for the reaction to occur.
Enzymes
Enzymes, which tend to be large protein complexes, catalyze reactions in biological systems.
- The exact catalysis mechanisms vary: e.g. changing the acidity or charge, or bending a molecule in a certain way, etc.
- Reactants (called the substrates) bind to the enzyme at certain active sites where the reaction then proceeds.
Consider the Enzyme Hexokinase shown in the schematic below .
Picture_Hexokinase with Magnesium Ion Co-factor
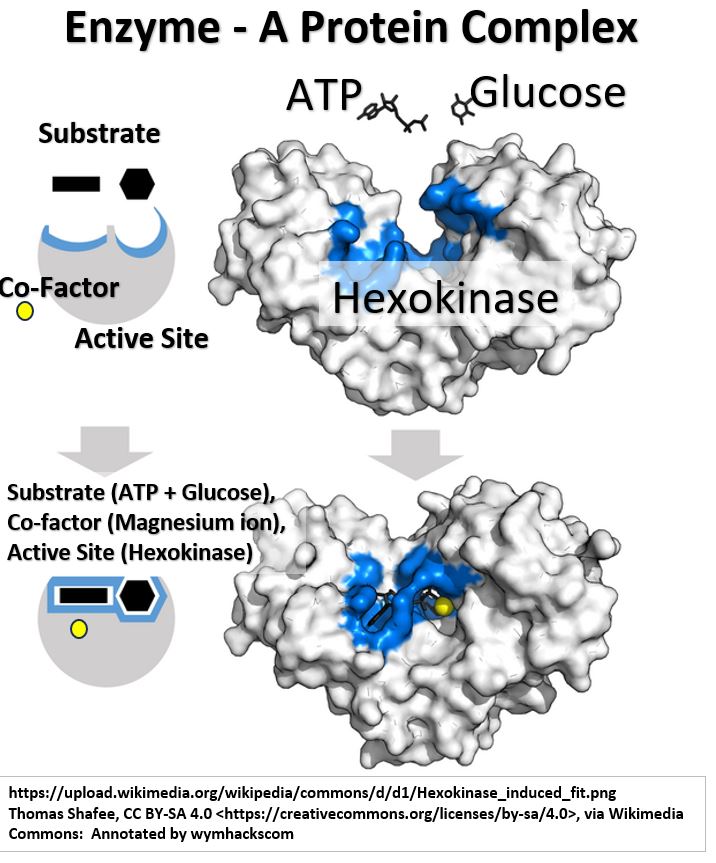
Hexokinase is a complex protein which provides active sites for reactants (substrates) to bind to and react.
- In this example, glucose is being phosphorylated by ATP (Adenosine Tri-Phosphate).
- Hexokinase actually changes shape when the substrates bind, enabling the reaction to proceed.
- You’ll often see enzymes symbolically shown as a circle with grooves on top showing binding sites for the reactants.
Notice the small yellow sphere in the schematic above.
- This is an additional molecule , called a Co-Factor, that will often be present and will also bind to the enzyme.
Co-Factors
In addition to the Enzymes themselves (where all the action is happening) , there are “actors” called Co-Factors that help enzymes catalyze reactions.
Organic Co-Factors are sometimes called Co-Enzymes. Vitamins and minerals often act as Co-Factors.
For example, Magnesium ions bind to Hexokinase near the active sites (see schematic above).
- They help draw electrons apart on the phosphate Oxygen atoms,
- therefore reducing electron repulsion and making it easier for the glucose-number-6-carbon oxygen to react with the “outer” phosphate of ATP.
Many critical biological reactions require Enzymes and the human body utilizes thousands of them.
So much interesting stuff to learn here. Check out these references:
Conclusion
More than 1,000 different types of proteins in the human body (called Enzymes) enable biological reactions to occur at physiologically required rates by reducing the Activation Energy of the reactions.
Co-Factors are chemical compounds that bind to an enzyme and assist in its catalytic function.
- Organic Co-Factors are called Co-Enzymes and they include Vitamins and chemicals like NADH, FAD, and Coenzyme A (all play a role in cell respiration / metabolism).
- Examples of Inorganic Cofactors are metallic ions like Iron (Fe2+) and Magnesium (Mg2+).
Enzyme Reference Material
- Activation Energy and Maxwell Boltzmann Distribution – Bozeman Science
- Enzymes – Khanacademy
- Activation-Energy – Document – Khanacademy
- Intro to Kinetics – Khanacademy
- Enzymes-and-the-Active-Site – Document – Khanacademy
- Enzyme-cofactors-and-coenzymes – Khanacademy
- Collision-theory – Khanacademy
- Arrhenius-equation – Khanacademy
- Collision-theory-Maxwell-Boltzmann-distribution – Khanacademy
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.
