Menu (linked Index)
ATP (Adenosine Triphosphate)
Last Update: October 26, 2024
Introduction
Adenosine Triphosphate (ATP) is the “energy currency” of the cell because it contains the energy needed to fuel cellular processes.
Cellular respiration is the process by which cells generate ATP by breaking down glucose and other molecules.
This post describes the structure of Adenosene Triphosphate (ATP) as well its hydrolysis by water.
ATP – Structure
You should appreciate the structure of ATP aka Adenosine Triphosphate (Tri-Phosphate)
ATP , along with the gene carrying Nucleic Acids, RNA and DNA, are some of the key chemical players in human biochemistry.
Picture_Adenosine Tri-Phosphate (ATP) C10H16N5O13P3
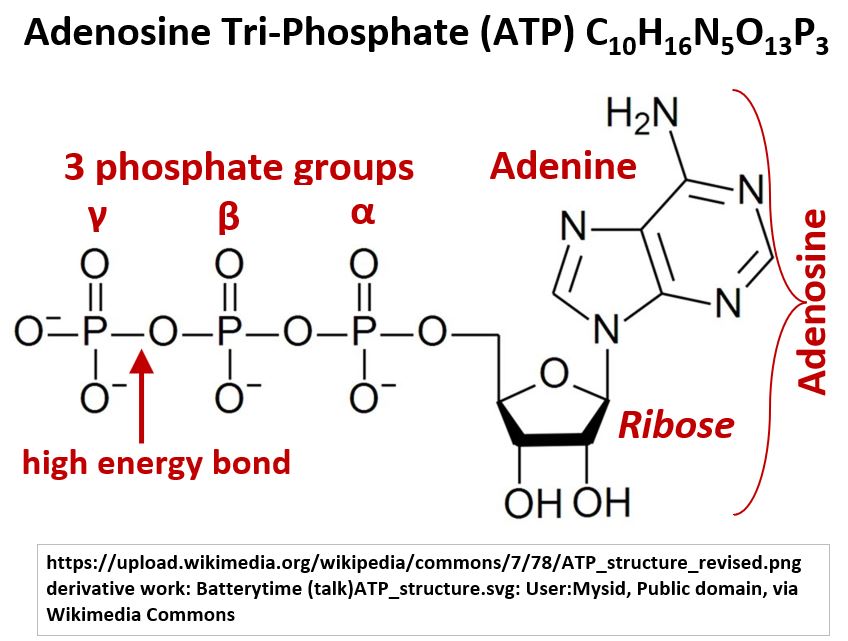
ATP is a Nucleotide that has 3 phosphate groups, a Pentose sugar (Ribose), and a nitrogenous Purine base (Adenine).
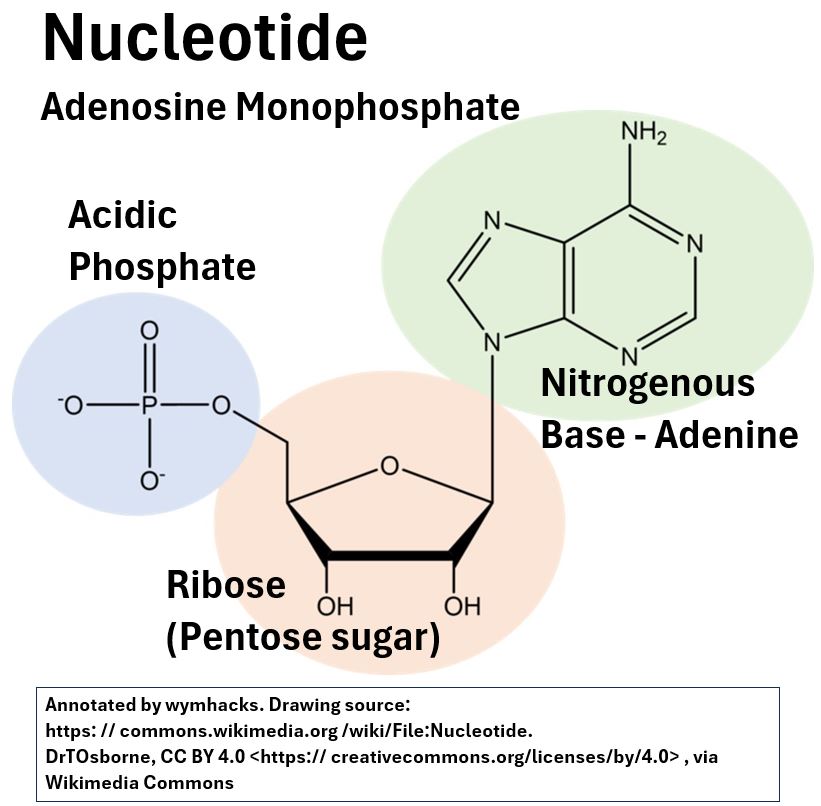
It is a three phosphate version of a mono-phosphate Nucleotide, Adenosene Monophosphate, that you would find in an RNA (RiboNucleic Acid) molecule (see the picture below).
- The AMP group is also found in ADP, NADH, and FADH2 (all play key roles in Cellular Respiration).
You can read more on nucleic acid structures and naming conventions in my post: Macro-Molecules, Minerals, and Vitamins; A Primer
Picture_An RNA Nucleotide
- ATP has three phosphate groups where the gamma bond is a very high energy bond which can release energy when broken.
- This gives ATP its high potential energy.
- The Adenosine part of ATP is a Nucleoside consisting of an Adenine nitrogenous base and a Ribose sugar.
- As we noted, ATP’s structure is very similar to an Adenine Nucleotide found in RNA (which has a single phosphate group).
- ATP’s structure is also similar to the Adenine DNA Nucleotide (with a single phosphate group and containing Deoxyribose sugar)
- So ATP (and other molecules involved in Cellular Respiration), RNA, and DNA are structurally very similar.
- Think about this: the key molecules that give your body energy and uniquely (genetically) define you just about look the same!
- In other words: Millions of years of natural selection (or whatever Grand Designer you Choose) have chosen (evolved) the following chemicals to be integral to your life functions:
- Phosphates
- Pentose Sugars (Ribose or DeOxyRibose)
- Nitrogenous Bases (Purines {Adenine, Guanine} or Pyrimidines {Cytosine, Uracil, Thymine})
- DNA Nucleotides contain DeOxyRibose sugar and the following Nitrogen Bases (Adenine, Thymine, Guanine, Cytosine)
- RNA Nucleotides contain Ribose sugars and the following Nitrogen Basis (Adenine, Uracil, Guanine, Cytosine)
See this great introductory video by Sal Khan:
ATP Hydrolysis and Phosphorylation Cycle
The body is constantly consuming energy from ATP hydrolysis and constantly making it via Cellular Respiration.
See my posts on Cellular Respiration starting with this one: Cellular Respiration (I) Overview
This ATP Hydrolysis and Phosphorylation cycle ensures a constant supply of energy for the cell’s needs.
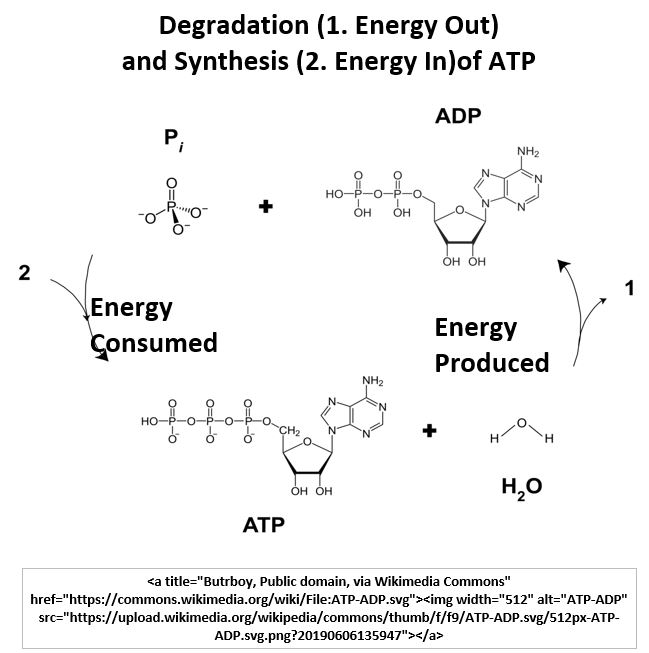
- ATP is hydrolyzed to ADP in the reaction ATP+H2O→ADP+Pi+ free energy where Pi is a phosphate group
- ADP is combined with a phosphate to form ATP in the reaction ADP + Pi+ energy→ATP+H2O.
Picture_ATP to ADP and ADP to ATP Reactions
Check out Khacademy’s videos on ATP hydrolysis
Conclusion
ATP, Adenosene Triphosphate is an RNA Nucleotide.
It is
- produced by Cellular Respiration Phosphorylation,
- and Hydrolyzed to produce energy required for our cellular processes.
The molecular structure of ATP contains a nitrogen base (Adenine) connected to a Pentose Ribose sugar which in turn is connected to three phosphate groups.
This Nucleotide structure and its various forms;
- Purine or Pyrimidine Bases attached to
- Pentose sugars (Ribose or DeOxyRibose) attached to
- up to three phosphate groups,
is found in biological molecules that play key roles in both body energetics and genetics.
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.