Linked Menu
Cellular Respiration Atomic Balance
Last Update: November 12, 2024
Introduction
- To twist your brain a little bit, we’ll also follow the electrons and try to confirm the number of water molecules predicted by the Cellular Respiration Equation.
Cellular Respiration Atomic Balance
Let’s count the carbons and hydrogens and oxygens in Cellular Respiration to see if they “balance” ie. all inputs = all outputs.
The chart below shows a top down high level sequential reaction pathway for Cellular Respiration starting with a mole of Glucose.
In my other articles on Cellular Respiration (see links below), I describe it as a four stage process because I (and others) count the Pyruvate to Acetyl CoA as a separate Transition Step.
In the drawing below, the Transition Step is (partially) included in the Krebs Cycle Box.
Picture_Cellular Respiration Atomic Balance
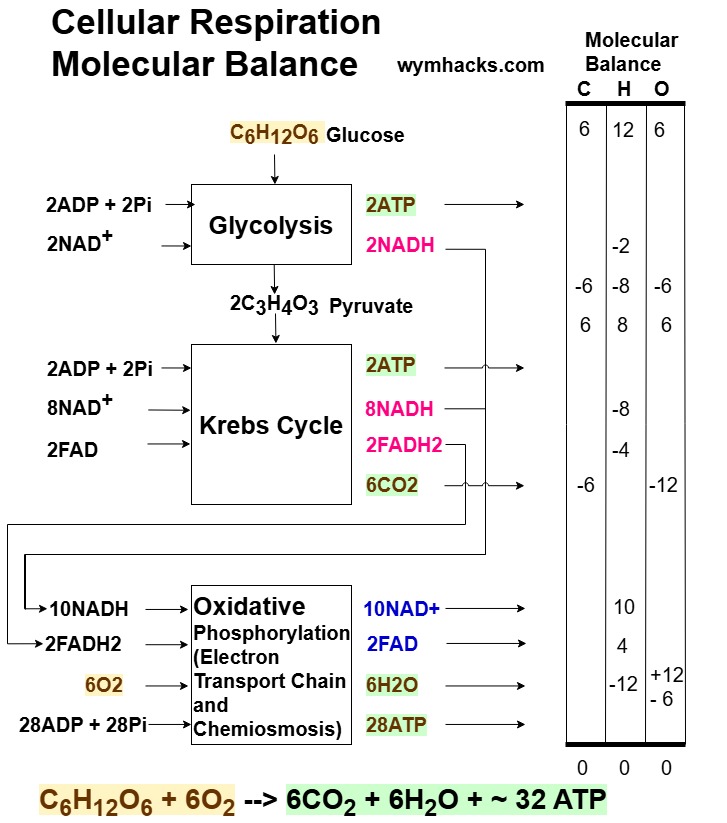
The balance is based on inflows (+) and outflows (-) around each “box” in the drawing above.
C, H, O Atom Balance
For example, around the last box (Oxidative Phosphorylation) we have
- 10 Hs coming in with the 10NADH
- 4 Hs coming in with the 2FADH2
- 12 Os coming in with the 6O2
- -12 Hs and -6Os coming out with 6H2O
- -6 Os coming out with
The full “tally” for each of the inflowing and outflowing Cs, Hs, Os should be zero for a balanced reaction.
If you count the columns of numbers for the C, H, and O atoms , you indeed get zero.
The table doesn’t include the ATPs, FAD, FADH2, NAD+, NADH but these balance as well.
Note: The literature does suggest some variability in exactly how many ATPs are produced so I’ve put a ~ in front of the 32 ATP in the overall Cellular Respiration Formula provided above.
So we’ve shown that we get a balanced series of reactions when 1 mole of Glucose results in:
- 32 ATP
- 10 NADH
- 2 FADH2
- 6 CO2
- 6 H2O
References
Video – Balanced Cellular Respiration – Andrew Douch
You can also check out my series on Cellular Respiration:
Cellular Respiration Water Balance
- Around 32 ATP,
- 10 NADH and
2 FADH2
Reduction / Oxidation of NAD+ / NADH and FAD / FADH2
Reduction / Oxidation of NAD+ / NADH
- NAD+ is reduced to NADH
- NADH is oxidized to NAD+
- Oxidation per molecule of glucose releases 10 x 2e- = 20 electrons
Reduction / Oxidation of FAD / FADH2
- FAD is reduced to NADH2
- FADH2 is oxidized to FAD
- Oxidation per molecule of Glucose releases 2 x 2e= = 4 electrons
What happens to the electrons after they pass through the Electron Transport Chain? They react to form water.
Water Production
The electrons from the Electron Transport Chain react with hydrogen and oxygen to form Water:
- 1/2O2 + 2H+ + 2e- → H2O
For 1 mole of Glucose, the balanced equation is :
- 6O2 + 24H+ + 24e- → 12H2O
But wait a minute, our overall Cellular Respiration Equation is
C6H12O6 + 6O2 —> 6CO2 + 6H2O + ~ 32 ATP
and says we make 6 moles of H2O (not 12).
Question: How do we reconcile the 12 H2O we predict with the 6 H2O of the Cellular Respiration Equation?
Water Balance Reconciliation
The Cellular Respiration Equation is the net overall accounting of atoms for all the steps of Cellular Respiration.
The 12 moles of H2O we calculated are based only on the final stage of this process (Oxidative Phosphorylation = Electron Transport Chain + Chemiosmosis).
So, we compute that 12 moles of water are produced (+) in the final stage which means 6 moles of H2O must be consumed (-) in the earlier stages of Cellular Respiration:
- 12 moles H2O produced – 6 moles H2O consumed = 6 moles net H2O produced.
This is where the waters (excuse the pun) get a little murky.
Glycolysis Water Production
According to “Lehninger-Principles-of-Biochemistry-Sixth-Edition-Chapter 14), the overall equation for Glycolysis (aerobic conditions) is:
Glucose + 2NAD(+) + 2ADP + 2Pi → 2 Pyruvate + 2NADH + 2H(+) + 2ATP + 2H2O
So, +2 moles of water are produced during Glycolysis of 1 mole of Glucose.
Citric Acid Cycle Water Consumption
According to “Lehninger-Principles-of-Biochemistry-Sixth-Edition-Chapter 16-Figure 16-7, in one cycle,
- Acetyl CoA to Citrate reaction involves (-)1 mole of water consumption
- Cis Aconitate reaction produces (+)1 moles of water
- Iso Citrate reaction consumes (-)1 moles of water
- Malate reaction consumes (-)1 moles of water
- For a Net Consumption of (-) 2 moles of water per Krebs cycle
For each molecule of Glucose , we have 2 Krebs cycles, so net H2O water use is -4.
Glycolysis + Citric Acid (Krebs) + Oxidative Phosphorylation Net Water
Adding up the above for one mole of Glucose Cellular Respiration:
- Net Glycolysis Water = + 2
- Net Citric Acid Cycle = – 4
- Net Oxidative Phosphorylation = + 12 H2O
- Total Net Water = 10 H2O
So back to our Question:
Question: How do we reconcile the 12 H2O we predict with the 6 H2O of the Cellular Respiration Equation?
Answer: We (I) Can’t. I compute a net of 10 H2O (not 6), so where are those 4 missing H2Os being consumed?
The only article I could find trying to explain this discrepancy is titled and referenced below.
- “Stoichiometry of the Water Molecules in Glucose Oxidation Revisited: Inorganic Phosphate Plays a Unique Role as Water in Substrate-Level Phosphorylation”
- Link to this Article via sciencedirect
- BIOCHEMICAL EDUCATION 24(l) 1996 ; KIHACHIRO HORIIKE,* RETSU MIURA,I_ TETSUO ISHIDA* and MITSUHIRO NOZAKI* , Department of Biochemistry Shiga University of Medical Science Seta, Ohtsu, Shiga 520-21 , and I-Department of Biochemistry Kumamoto University School of Medicine Honjo, Kumamoto, Kumamoto 860 Japan
According to the paper
- There are an additional two waters(-2) being consumed during Glycolysis and
- an additional two waters (-2) being consumed during the Citric Acid Cycle.
- These (-) 4 additional H2Os consumed occur during the Substrate Level Phosphorylations (i.e. the non Oxidative Phosphorylations) that produce ATP from ADP in the Glycolysis and Citric Acid Cycle steps.
Sadly, I don’t think I can adequately explain what is really going on here more that what I wrote above.
You’ll have to read the paper yourself to see if you can get it clear in your head.
If you get it and think you can explain it to an educated but not necessarily expert audience, then send me a comment with your explanation.
Conclusion
So, breath calmly and sleep well.
The Atoms balance and the Universe is Knowable.
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.
