Menu (linked Index)
Cellular Respiration (V) Oxidative Phosphate Addition (Phosphorylation)
Last Update: October 30, 2024
Introduction
This is part V of a 5 part series on Cellular Respiration.
You can access the other posts via the links below.
- Cellular Respiration (I) Overview
- Cellular Respiration (II) Glycolysis
- Cellular Respiration (III) Pyruvate to Acetyl CoA
- Cellular Respiration (IV) Krebs Cycle
- Cellular Respiration (V) Oxidative Phosphorylation
The fourth and final stage of Cellular Respiration culminates in the production of about 27 to 32 molecules of ATP.
This occurs via Oxidation of NADH and FADH2 and Phosphorylation of ADP (Adenosene Diphosphate) to ATP (Adenosene Triphosphate).
We are going to dig into this , because it presents stark and mind boggling evidence that the Cosmos is orderly and knowable (sorry Nihilists).
Cellular Respiration Makes a lot of ATP
The final stage of Cellular Respiration (the 4th Stage according to the way I’ve organized the sequences), generates most of the cell’s ATP.
Remember that ATP is the energy currency of the body.
By releasing a phosphate group and going to ADP, ATP releases a large amount of useful energy
- ATP is hydrolyzed to ADP in the reaction ATP+H2O→ADP+Pi+ free energy where Pi is a phosphate group
- The body uses this energy for numerous cellular tasks which
- keep you alive.
See the picture below showing where cellular respiration actually occurs in the human cell (in most human cells).
Oxidative Phosphorylation – Overview
Picture_Cellular Respiration Locations in the Human Cell
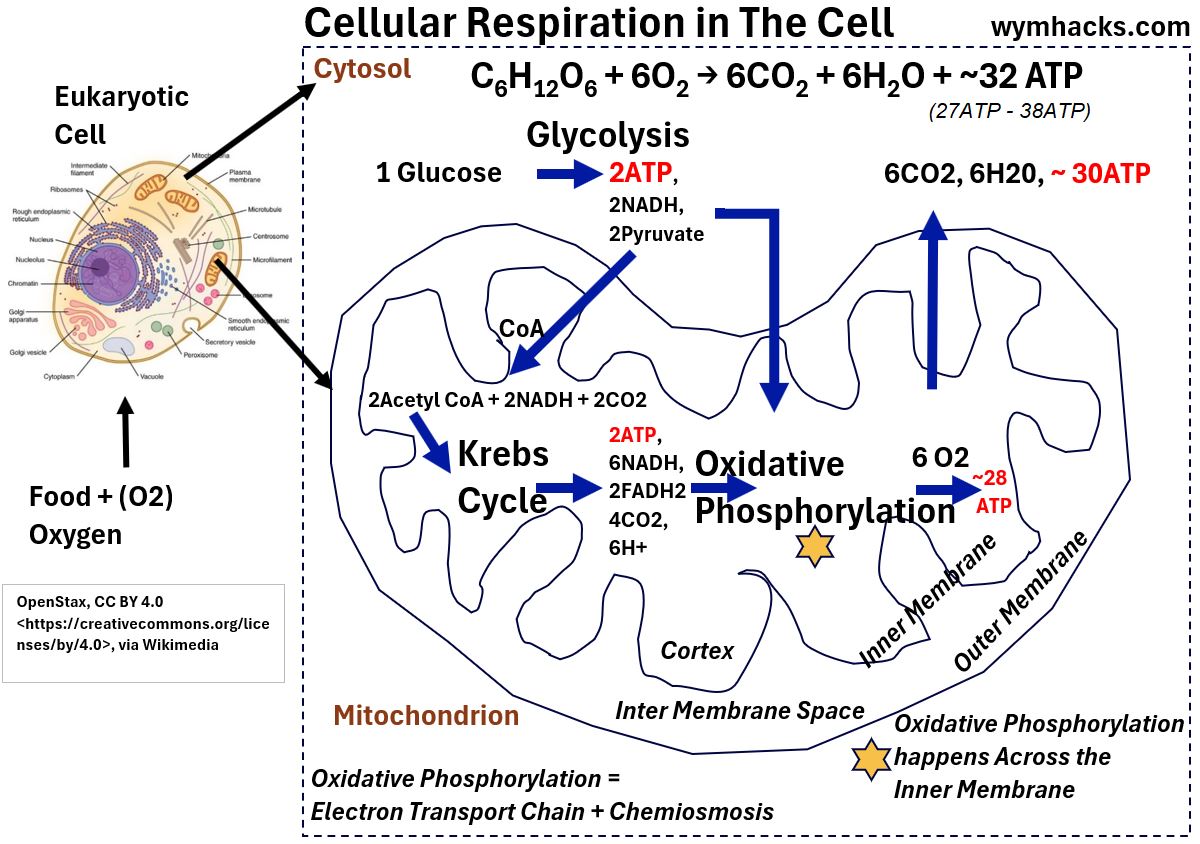
- The electron carriers NADH and FADH2 play a major role in the production of ATP.
- Electrons in the NADH produced by Glycolysis in the cell Cytoplasm are shuttled into the Mitochondrial Matrix
- where they join the electron carrying molecules NADH and FADH2 (produced by the Krebs Cycle).
- By an amazing electro-chemical-mechanical process,
- the NADH and FADH2 are oxidized , releasing electrons.
- The electrons move through a series of protein molecules (Electron Transport Chain) which are energized.
- The energized molecules kick off a chemiosmotic process that ultimately results in the Phosphorylation of ADP to ATP.
- Depending on the efficiency of the electron transfer from NADH and FADH2,
- about 30 – 32 ATP are produced.
- Assuming 2.5 ATP/NADH and 1.5 ATP/FADH2
- about 32 ATP are made for each molecule of Glucose.
Oxidative Phosphorylation occurs across the inner membrane of the Mitochondrion.
- See the inner membrane in the picture where the orange star is placed.
We have to take closer look at this.
Oxidative Phosphorylation Steps
Picture_Oxidative Phosphorylation: Electron Transport Chain and Chemiosmosis
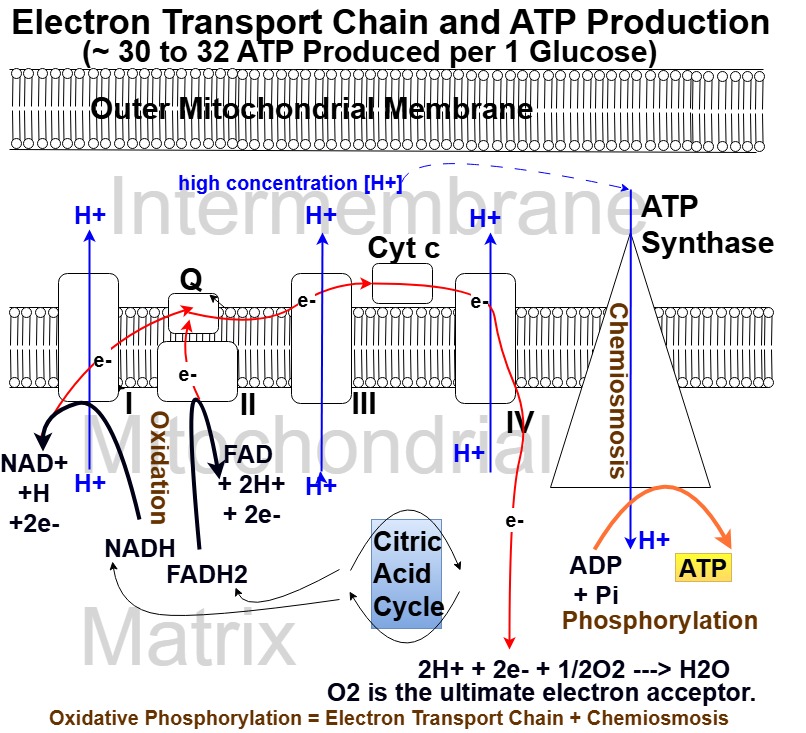
- High energy electron carrying NADH and FADH2 enter the Mitochondrial Matrix.
- They come from the Krebs cycle and indirectly from Glycolysis.
- These electron carriers are Oxidized and pass their electrons down a series of proteins embedded in the mitochondrial membrane.
- The pathway of these electrons is called the Electron Transport Chain.
- The process is driven by various enzymes.
- There are 6 proteins involved in this electron cascade,
- 4 of which are considered complex and
- consist of many protein subunits (Complexes I,II, III, IV).
- The remaining two, Q and Cytochrome C, are simpler proteins but are key in facilitating the movement of electrons.
- As electrons move, they release energy, energizing Complexes I, III, IV to pump protons (H+) across the membrane.
- The resultant proton gradient drives the manufacture of ATP by Chemiosmosis of the protons.
- The Chemiosmosis drives the chemo-mechanical actions of the enzyme ATP Synthase which Phosphorylates ADP to ATP.
- NADH and FADH2, after being Oxidized and giving up their electrons, are regenerated and head back to the Krebs cycle to repeat the process.
- That is , NADH–>NAD+ and FADH2–>FAD
- NAD+ and FAD are the oxidized forms of these molecules
- In the absence of oxygen (anaerobic), Pyruvate helps regenerate NADH to NAD+
- When oxygen is scarce, NAD+ regeneration is limited in the electron transport chain.
- In anaerobic conditions, pyruvate acts as an electron acceptor and generates NAD+ from NADH.
- Lactic Acid is also produced.
- This whole process is called Metabolic Fermentation
Protein Complex Actions
Let’s go through the steps again with a focus on the protein complexes.
- Electrons transfer through the protein complexes
- They are complex in that they are composed of many types of protein units that assume various unique shapes.
- Protein Complex I: Receives electrons from NADH (NADH is oxidized to NAD+).
- It contains flavin mononucleotide (FMN – derived from Vitamin B2) and iron-sulfur (Fe-S)-containing proteins.
- It helps to establish a hydrogen gradient (i.e. one side has more than the other side)
- across the inner membrane by pumping hydrogen ions across the membrane.
- Protein Complex II receives electrons from FADH2 and allows them to flow to Coenzyme Q.
- It contains a Vitamin B2 derivative and iron-sulfur clusters as well
- Coenzyme Q (ubiquinone) connects the complex I and II electron flow.
- Protein Complex III pumps protons to the intermembrane space and passes electrons to Cytochrome C.
- Protein Complex IV accepts electrons from Cytochrome C
- and is also energized to pump more hydrogen into the intermembrane space.
- Complex IV is the terminal electron acceptor and combines the electrons with oxygen to form water.
2H+ + 2e + 1/2 O2 ——-> H20
So, the electron cascade ultimately terminates when the electrons (along with hydrogen) react with oxygen to form water.
In other words, oxygen, the final electron acceptor in the Electron Transport Chain is reduced and forms water.
Proton Gradient
The
- energizing of the electron transferring proteins
- and the subsequent pumping of protons into the intermembrane space of the Mitochondria,
- create a concentration gradient (higher concentration in the intermembrane space).
- Since the protons are charged, an electrical gradient is also created.
- To imagine both phenomena, think of a rising potential energy that will be released if there is a path to a lower energy state.
- e.g. like water behind a dam.
The pathway to release this gradient is the enzyme ATP synthase.
ATP Synthase (Chemiosmosis)
Due to the presence of an electrochemical gradient (i.e. concentration and charge is higher on one side than the other side) ,
- the hydrogen will tend to want to flow to the Matrix if there is a path to do so.
- The inner membrane is impermeable to the H+.
- ATP synthase embedded in the inner membrane, allows this to happen.
It is truly a rock star chemical belonging up there with DNA, RNA, ATP and a few others.
Picture_ATP Synthase
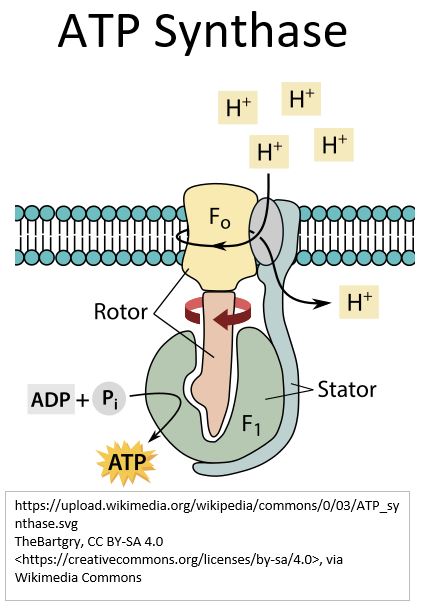
How ATP Synthase works
ATP Synthase operates like a water or gas turbine.
It permits hydrogen ions (protons) to flow back to the Matrix in a process called Chemiosmosis.
- ATP Synthase is a complex enzyme.
- It contains Iron-Sulfur clusters and
- metal ion Magnesium (Mg2+ form) that are crucial to its proper functioning.
- It consists of two main parts.
- Fo is a shaft that rotates via the flow of protons.
- F1, located in the Mitochondrial Matrix, is the catalytic site where ATP is manufactured.
- The Fo shaft rotation causes shape changes in the reaction section F1.
- These changes cause the formation of ATP from ADP and also cause the release of newly minted ATP into the Matrix.
- So, to summarize,
- the spinning shaft within ATP Synthase has used the energy from flowing protons
- to attach a phosphate group to ADP,
- creating ATP (the powerhouse chemical that we need for life).
Check out this amazing video of ATP Synthase in action: Electron Transport Chain – Harvard Video of ATP Synthase
Summary of Electron Transport Chain and Oxidative Phosphorylation
Through an amazing symphony of carrier molecules, enzymes, coenzymes, and protein complexes,
- the Electron Transport Chain, harnessing the energy produced by Glycolysis and the Krebs Cycle,
- produces the powerhouse ATP molecules that make our lives possible.
- One cycle of Glycolysis and the Krebs cycle produces 4 ATP and 10 NADH and 2 FADH
Oxidation
- The NADH and FADH2 in the Mitochondrial Matrix are Oxidized, donating electrons to fuel the Electron Transport Chain.
- This occurs in and across the inner membrane of the Mitochondria.
- The donated electrons cascade through a series of proteins and protein complexes, releasing energy in the process.
- The released energy activates/energizes the Protein Complexes.
- Energized Protein Complexes I, III, and IV pump hydrogen ions into the intermembrane space
- creating an electrochemical Hydrogen ion (proton) gradient.
- Ultimately the electrons react with oxygen to form water.
- Oxygen is reduced and water is produced.
- Oxygen is the final electron acceptor in the Electron Transport Chain.
Chemiosmosis and Phosphorylation
- The enzyme ATP Synthase, in a process called Chemiosmosis, allows hydrogen ions to flow through it into the Matrix.
- A shaft mechanism in ATP Synthase is rotated by the flow of Protons,
- allowing for phosphate groups to react with ADP (Phosphorylation) to form ATP.
- The ATP is released into the Matrix.
ATP Count
- The literature is not 100% consistent on the number of ATP produced per molecule of NADH and FADH2 (it’s an ongoing area of research).
- The following numbers are based on Khanacademy.org , NIH and a few others:
- About 2 to 3 ATP per NADH2 (so “10 x 2 = 20” to “10 x 3 = 30” ATP produced)
- About 1.5 to 2 ATP per FADH2 (so “2 x 1.5 =3” to “2 x 2 =4” ATP produced)
- So via the Electron Transport Chain about 23 to 34 ATPs are produced.
- Counting the 4 ATP produced directly from Glycolysis and the Krebs Cycle,
- one cycle of cellular respiration starting from Glucose produces 27 to 38 ATP.
- Sometimes 30 – 32 is suggested as the actual range.
Conclusion
The majority of ATP, the powerhouse cell of the body, is produced by Oxidative Phosphorylation. This occurs in the final stage of Cellular Respiration.
The Oxidation occurs to the electron carriers NADH and FADH2 which are produced in the earlier phases of Cellular Respiration.
The released electrons move through a series of proteins , resulting in the creation of a proton gradient between the Mitochondrial Matrix and the Intermembrane of a Mitochondrion.
At the end of the electron cascade, electrons react with hydrogen and oxygen to form water.
Through Chemiosmosis of the protons through the enzyme ATP synthase, ATP is produced from the Phosphorylation of ADP.
For every molecule of Glucose about 27 to 38 molecules of ATP are formed.
Here are some nice references on Oxidative Phosphorylation:
Videos
- Oxidative phosphorylation and the electron transport chain-khanacademy.org
- Electron transport chain-khanacademy.org
- Oxidative phosphorylation and chemiosmosis-khanacademy.org
- ATP synthase-khanacademy.org
- Calculating ATP produced in cellular respiration-khanacademy.org
Documents
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.
