Menu (linked Index)
Macro Molecules, Minerals, and Vitamins – A Primer.
Last Update: October 27, 2024
Introduction
This article will give you a high level introduction to Macro Molecules as well as Vitamins and Minerals.
Macro Molecules are large (macro) molecules composed of many atoms. Be aware that the term “large” is relative. A Macro Molecule, might ,for example. still be millions of times smaller than a grain of rice.
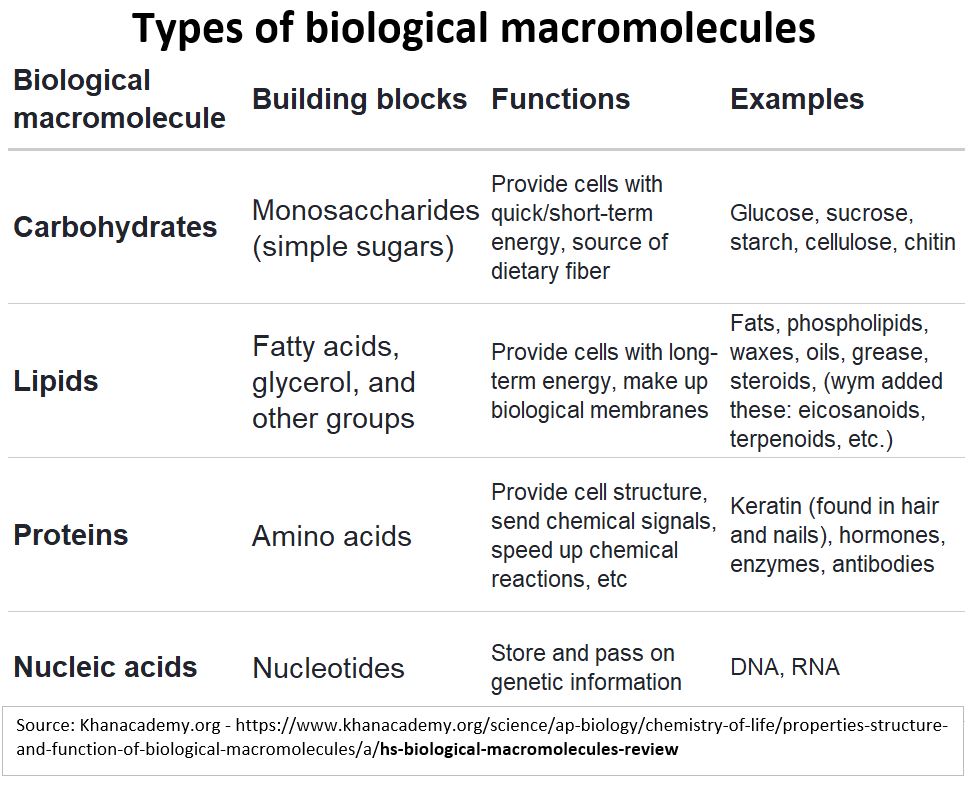
Macro Molecules are typically organized into one of four categories:
- Carbohydrates,
- Proteins,
- Lipids,
- Nucleic Acids.
Another important category of molecules are Vitamins and Minerals which are essential nutrients your body needs but cannot make. So You have to get these through your diet.
Be Aware of the following:
-
- We are referring to classes of molecules inside the body.
- Often you will hear the term Macronutrient and that refers more to the general categories of food we ingest (Carbohydrates, Proteins, Fats).
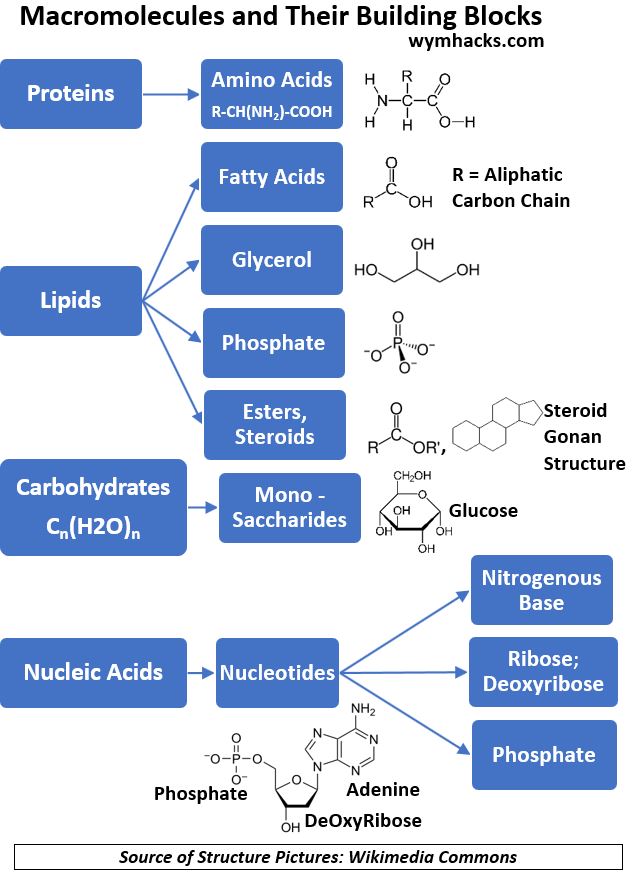
The Chart and table below categorize the various macronutrients we’ll discuss in the following sections.
Chart_Macro Molecules and Their Building Blocks
Carbohydrates
Carbohydrates are sugar molecules (in the form of simple sugars, or more complex sugars like Starch or Fiber) and are produced from plants via photosynthesis.
They are a major fuel source for our bodies (especially brain and central nervous system).
- Carbohydrates are Macro Molecules (very large compared to small molecules like water) that contain carbon and oxygen and hydrogen where the ratio of H to O is 2:1 (like water).
- Your body breaks down carbohydrates into simple sugars (monosaccharides), the most important one being glucose (C₆H₁₂O₆).
- Glucose is “the shit” (much sweeter though). It’s one of the most important molecules needed for life.
- Glucose is a sugar (aka saccharide; it’s sweet). It’s the primary way your body gets the energy it needs to survive.
- Glucose can polymerize to form a larger molecule called glycogen. The body stores glucose as glycogen.
- There are lots of other sugars: Monosaccharides (glucose, fructose, galactose), Disaccharides (sucrose, lactose, maltose) etc.
- Glucose can assume a linear molecular structure, but ,in our bodies, glucose typically has a cyclic structure (mostly the Beta form).
Picture_Glucose – Alpha-D-Glucose and Beta-D-Glucose
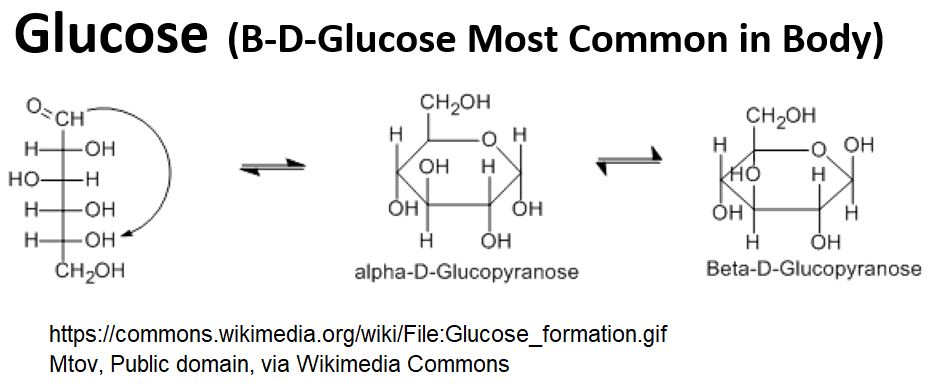
- Glucose levels in your blood are tightly controlled (if you are healthy and don’t have Insulin Resistance or worse, Diabetes)
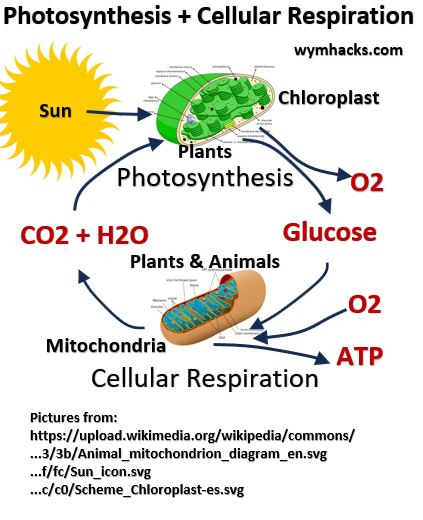
- Photosynthesis in plants makes glucose:
- 6 CO₂ (carbon dioxide) + 6 H₂O (water) + Sunlight = C₆H₁₂O₆ (glucose) + 6 O₂ (oxygen).
- Photosynthesis is an example of an anabolic process where energy (e.g. sunlight) is used to build up new molecules (glucose from CO2 and H20).
- Cellular Respiration in Humans utilizes glucose for energy:
- C₆H₁₂O₆ (glucose) + 6 O₂ (oxygen) = 6 CO₂ (carbon dioxide) + 6 H₂O (water) + ATP (energy)
- Cellular Respiration is an example of a catabolic process where components are broken down and energy is extracted.
- Glucose plays a central role in both Photosynthesis and Cellular Respiration
Picture_Photosynthesis and Cellular Respiration Cycle
Proteins
Proteins, made up of amino acids, build and repair tissues but also perform many other functions in the body.
Their function is often related to their complex shapes which are the result of chemical interactions among their amino acid chains.
- Proteins are also very large Macro Molecules.
- For example, some proteins might be 100s of thousands of times larger than a hydrogen molecule
- The word “protein” originates from the Greek word meaning “primary,” “first place,” or “standing in front” due to the prominent role proteins play as building blocks of living organisms.
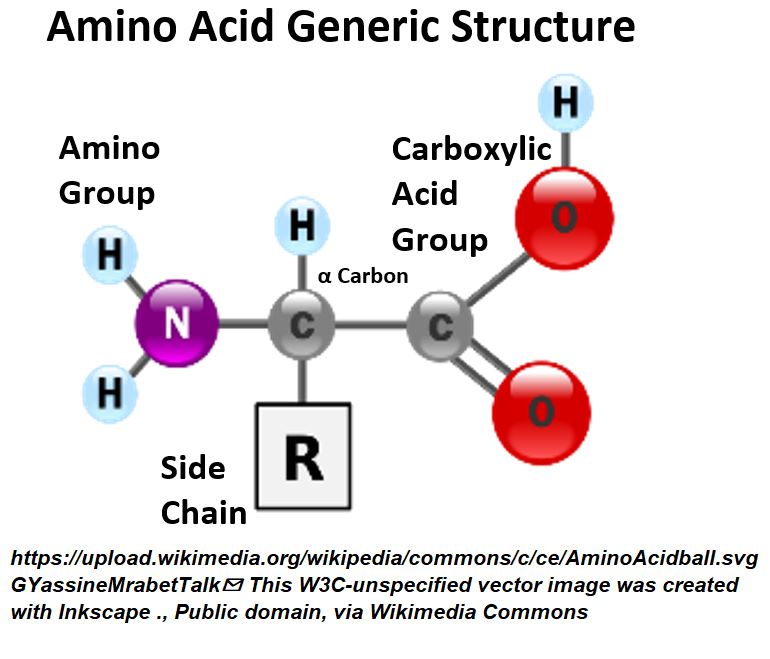
- Proteins comprise single or multiple chains of chemicals called amino acids.
- These protein polymer chains are called Polypeptides.
Picture_Amino Acid Structure
Proteins have many roles in the body. Some examples are:
-
- Provide Structure (in cells, tissues, and organs)
- Have a Mechanical role. e.g. Actin and Myosin in muscle contraction.
- Act as Enzymes (catalyze reactions by reducing their Activation Energies)
- Involved in Signaling and Regulating (sent from one part of body to another). Some Hormones are composed of protein (e.g. Insulin, Glucagon, Growth Hormone, Thyroid Stimulating Hormone).
- Involved with Immune System (e.g. Antibodies)
- Can be Receptors , Carriers, and Channels
- There are over 500 amino acids in nature but only 22 are proteinogenic (used to build proteins in living things).
- Of the 22 proteinogenic amino acids, only 9 are considered Essential; meaning the body cannot synthesis these amino acids and must get them from diet.
Table of Amino Acids: 22 Proteinogenic Amino Acids; 9 Essential Amino Acids
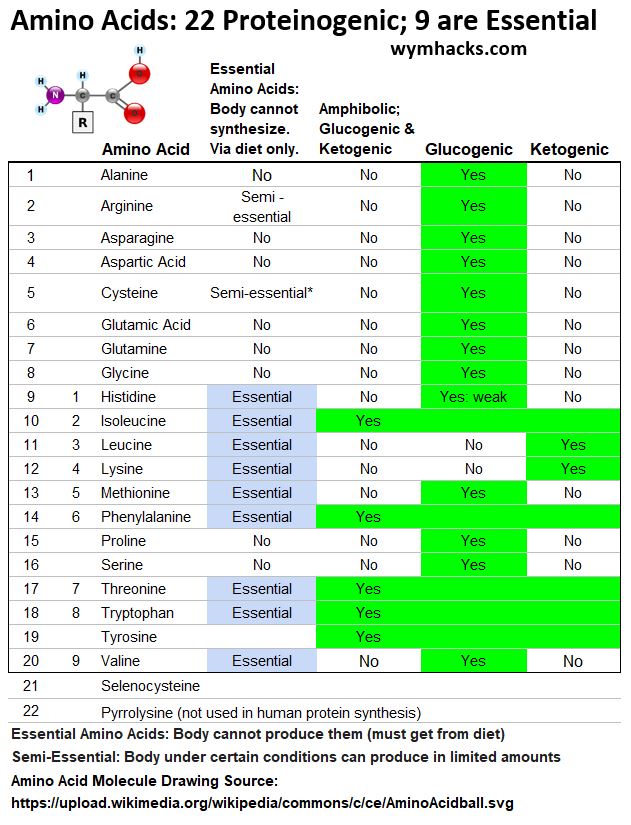
- Glucogenic Amino acids can be converted to Glucose.
- Ketogenic Amino Acids can be converted to Ketone Bodies.
Lipids
Lipids, of which Fats are a subcategory, are a broad grouping of M acromolecules which have the general characteristic of being “not completely water soluble” (i.e. they are at least partially hydrophobic).
Because of the broad definition, the types of building blocks that make up lipids can vary quite a bit.
Here are some nice overviews of lipids:
- Lipids – The Organic Chemistry Tutor
- Lipids Intro – khanacademy
- Lipids Overview – khanacademy
- Lipids – Document –khanacademy
Lipids have multiple critical roles in our bodies.
- Lipids are the main form of long term energy storage in the body.
- They are major components of cell membranes and help control movement in and out.
- Lipids provide insulation (maintaining core temperature) and protection (to our organs for example).
- They are building blocks for Steroid Hormones.
- Fats are a type of lipid but not all lipids are fats (i.e. a fat is one type of Lipid).
- In terms of dietary macronutrients we would typically be talking about Fats.
- Lipids are a class of Macro Molecules which have the characteristic of being not completely hydrophilic (loving water; aka water soluble).
- Lipids all tend to (nut not always) have long tails of hydrophobic (water hating; don’t dissolve in water) carbon-hydrogen chains.
- Lipids might also have hydrocarbon ring structures which also will be non polar (i.e. wont dissolve readily in water).
Picture_Triglyceride, Phospholipid and Cholesterol Lipids
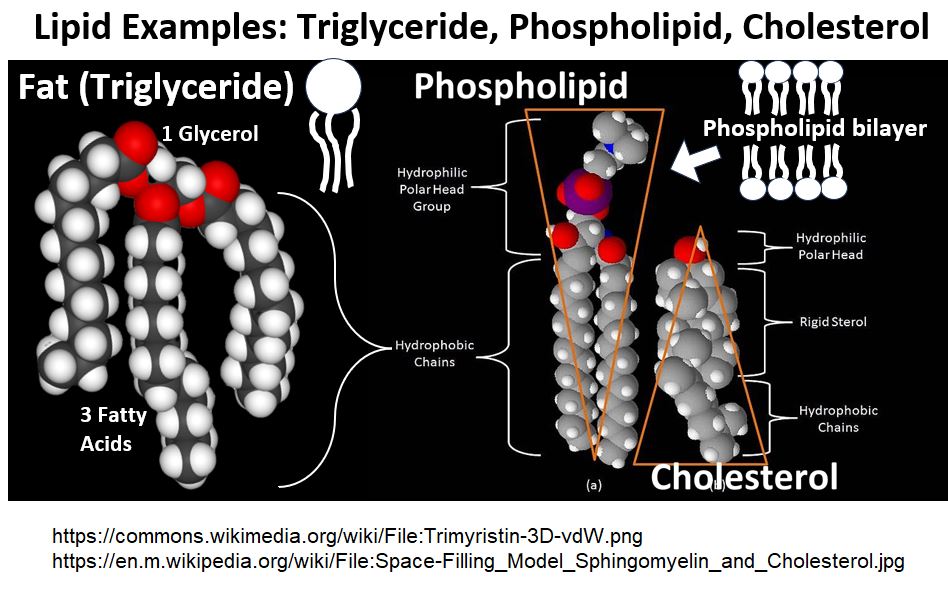
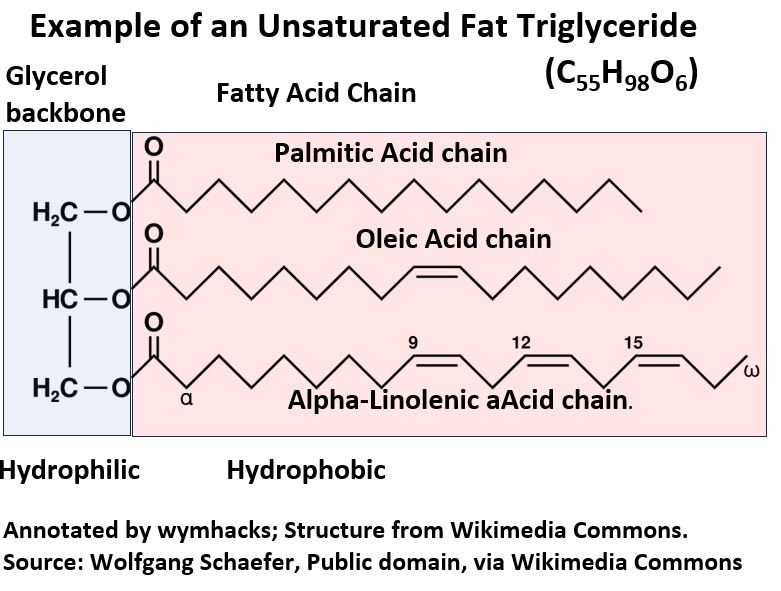
In the drawing above (and below), notice that Fat has a Triglyceride structure (it has a Glycerol backbone with three Fatty Acids groups attached to it).
Picture_Triglyceride (Fat) Example
- As we’ve noted, fat is not the only type of Lipid.
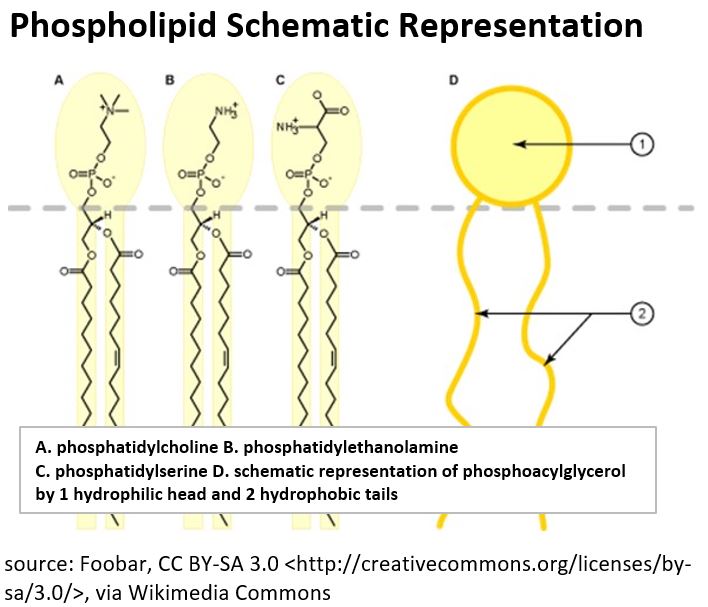
- Phospholipids for example, are Lipids that are major components of our cell membranes (which take on phospholipid bilayers with the hydrophobic tails tucked on the inside).
- Phospholipids contain two hydrophobic fatty acid chains, and a hydrophilic “phosphate attached to a nitrogen group”
Picture_Phospholipid
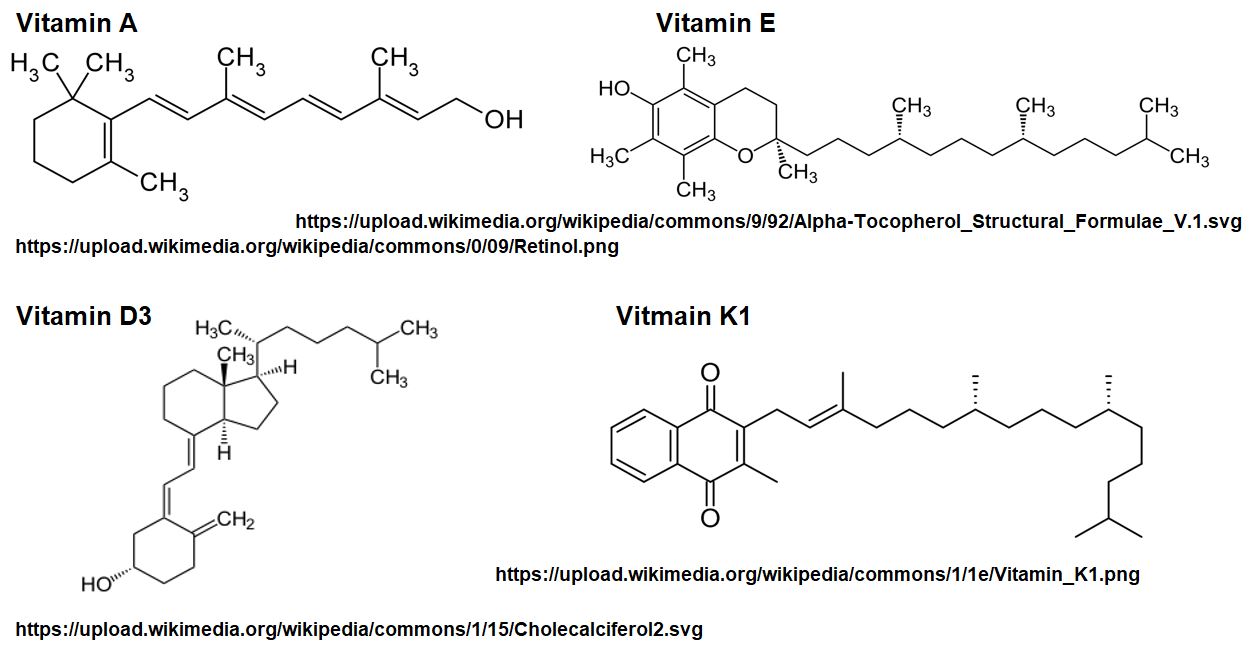
Picture_Vitamin A D E K Lipid Structures
- Steroids are another important class of Lipids. They all share a common hydrophobic multicyclic fused structure.
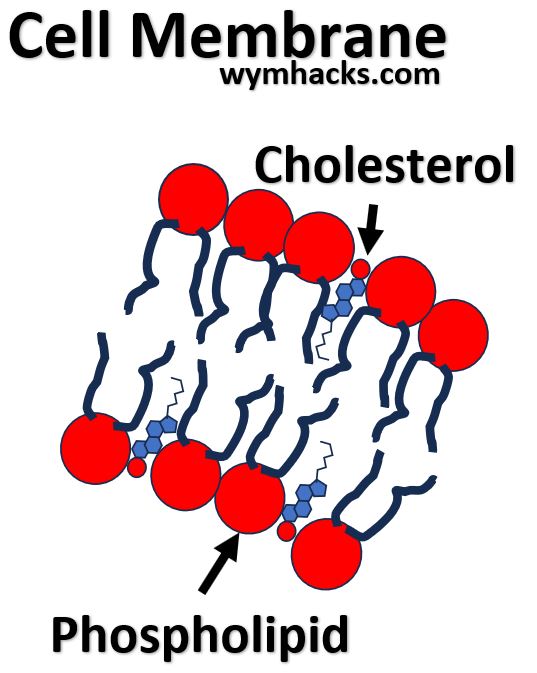
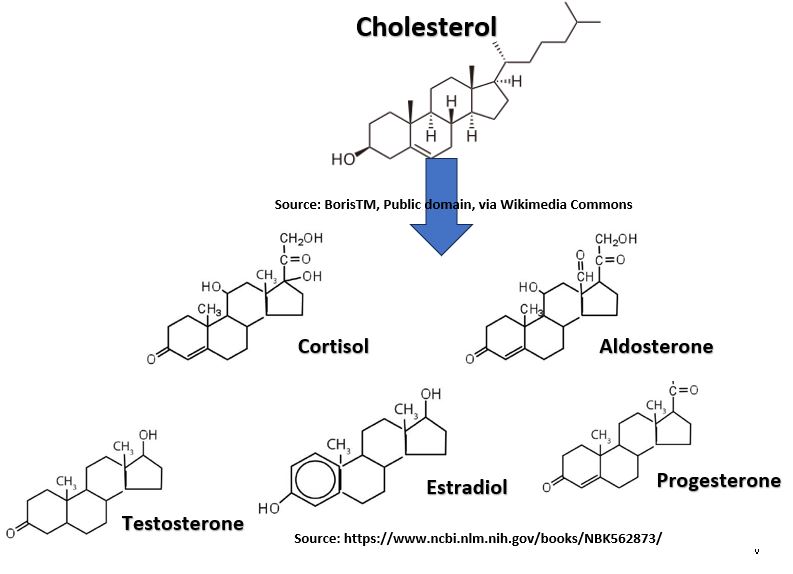
- Cholesterol is probably the most well known Steroid. It has multiple roles in our body.
- Cholesterol is part of cell membranes and provides fluidity to the membrane structure.
Picture_Cholesterol in Cell Membranes
-
- Cholesterol is a precursor molecule for several steroid hormones (estrogen, testosterone, cortisol etc.).
Picture_Cholesterol_Derivatives
-
- It reacts with sunlight UV to produce Vitamin D in the skin.
- Cholesterol is contained in Bile, a fluid produced by the liver and stored in the gallbladder (helps with the digestion and absorption of fats).
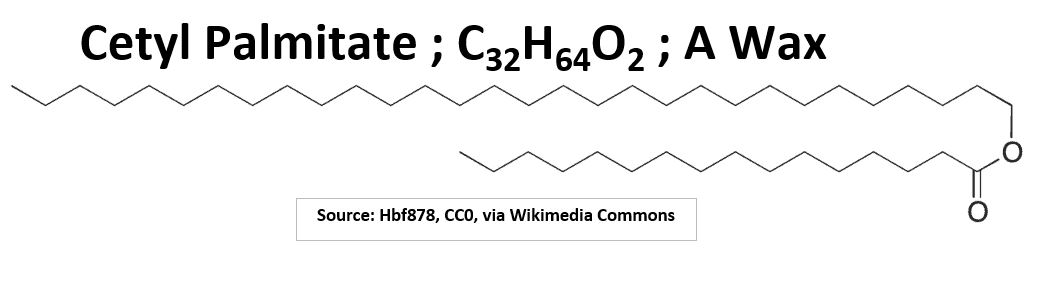
- Waxes are also lipids. These are esters of long chain alcohols and fatty acids.
Picture_Cetyl Palmitate A Lipid Wax Example
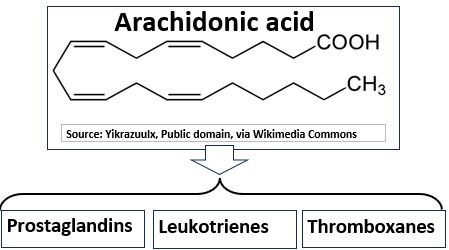
- Eicosanoids are a class of signaling molecules. They are also lipids and are key players in our immune systems.
- Injury, infection, and hormonal changes can cause Arachidonic Acid, a polyunsaturated fatty acid (PUFA), to be enzymatically converted into an array of biologically active compounds called Eicosanoids.
- Eicosanoids include Prostaglandins, Thromboxanes, and Leukotrienes.
- They are involved in regulating inflammation, blood clotting, and immune responses. They are involved in reproduction as well.
Picture_Arachidonic Acid. A lipid and Progenitor of Eicosanoids
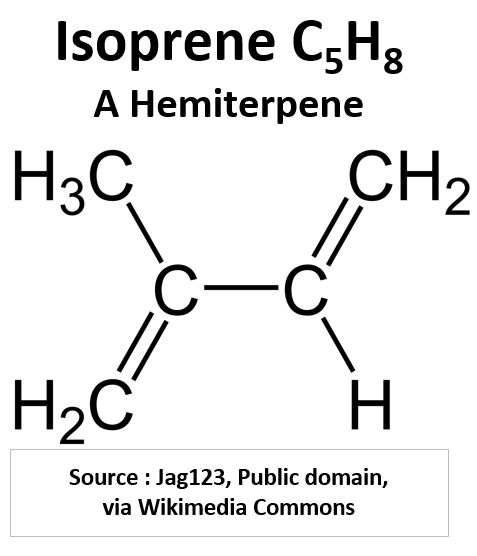
- Terpenes make up a huge number (>30,000) of molecules, most coming from plants. They tend to have repeating units of isoprene which is a 5 carbon diene (two sets of double bonds).
- Many plant essential oils are Terpenes.
- Terpenes can be described as having the general formula (C5H8)n but this wont always be true depending on the functional groups that are part of the structure.
Picture_Isoprene (C5H8)
To count the number of repeating C5 units,
- Count carbons 1 through 4 with the methyl group coming off of carbon 2 (always off of carbon 2).
- Know that they (the C5 groups) will connect in three ways (1 carbon of one group to 4 carbon of other group, or 1 carbon to 1 carbon, or 4 carbon to 4 carbon)
- Watch this video on how to do this: Recognizing Terpenes
Picture_Terpene Examples
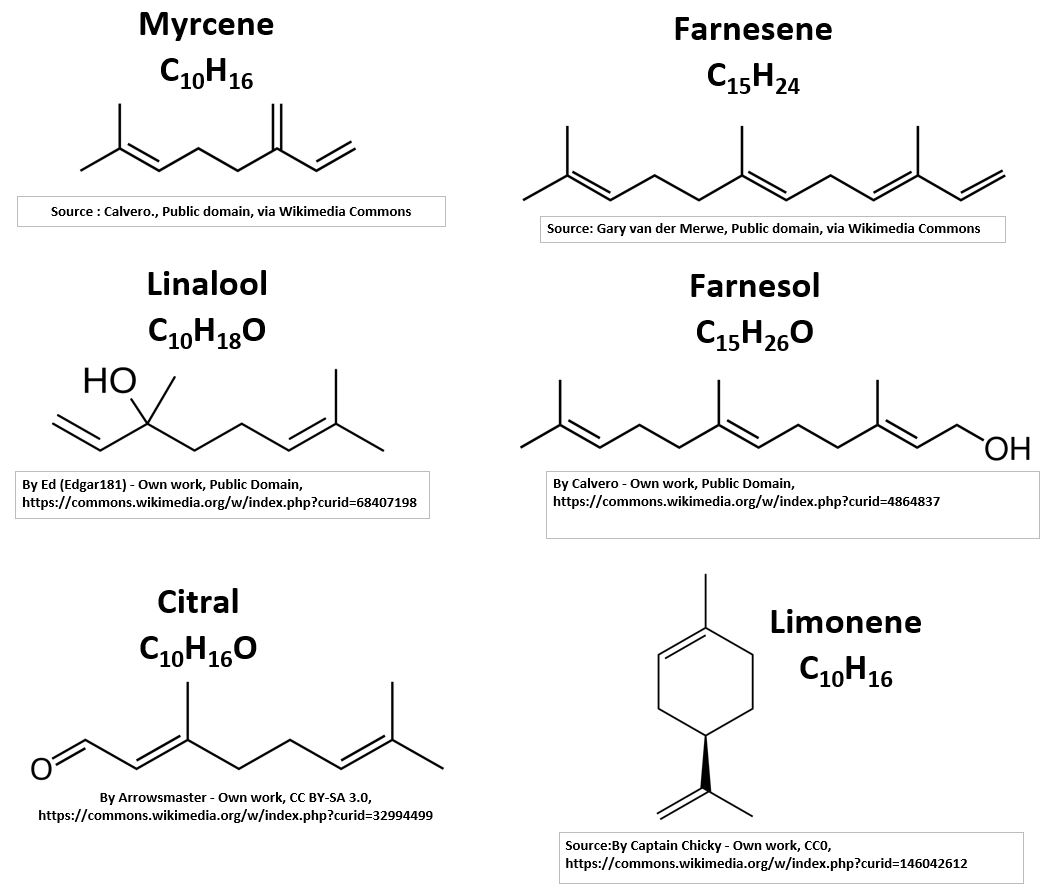
The Terpene examples above are a very small sampling of the known Terpenes (over 30,000 of them!). They have diverse uses as you can see from the list below (look at the chart above for their structure).
Example Terpene Descriptions
- Myrcene C10H16 or (C5H8)2 – Used in fragrance and food flavoring.
- Linalool C10H18O -has uses in manufacturing of soaps, fragrances, household products etc.
- Citral C10H16O – has a strong lemon (citrus) scent and is used in manufacturing perfumes.
- Limonene C10H16 – is the major component in the essential oil of citrus fruit peels.
- Farnesene C15H24 – is found in the coating of apples, and other fruits.
- Farnesol C15H26O – is present in many essential oils and is used in perfumery (sweet and floral).
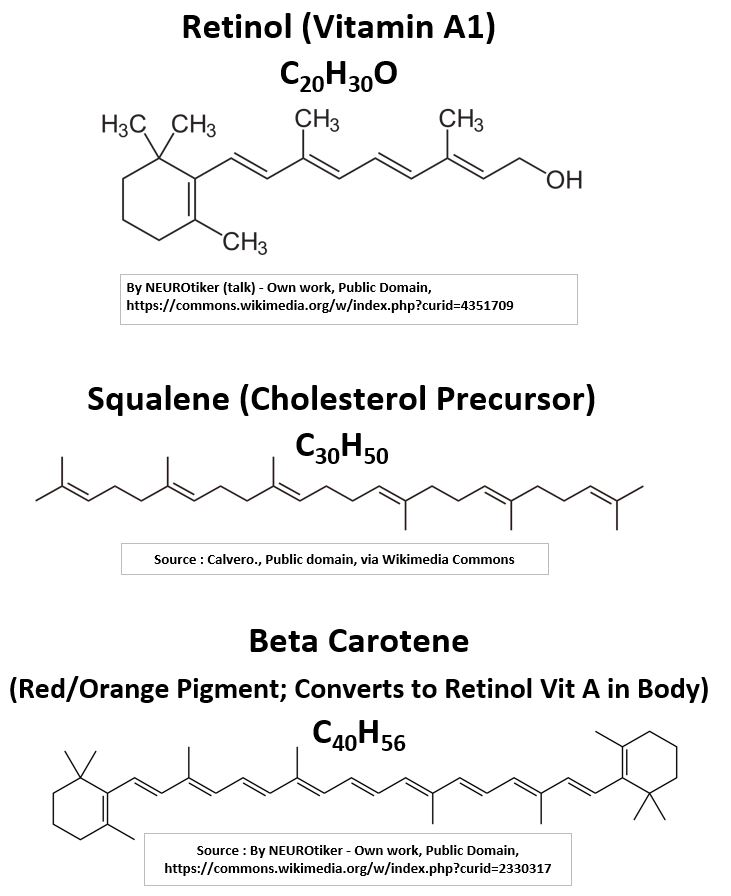
- Retinol C20H30O – (Vitamin A) is a Terpene.
- Beta Carotene C40H56 – found in plants and fruits and is converted to Retinol (Vit. A) in the body.
- Squalene C30H50 – The body manufactures cholesterol from squalene (which itself is built from isoprene building blocks).
Nucleic Acids
Nucleic Acids get their names from being discovered in the nucleus of cells (watch this and this to learn about our amazing cell structures ).
Your DNA (and RNA), the molecules that hold and “transmit” your genetic code, are nucleic acids.
- DNA (DeOxyRiboNucleic Acid) and RNA (RiboNucleic Acid) molecules are the major molecules that hold and express your traits (your genetic identity).
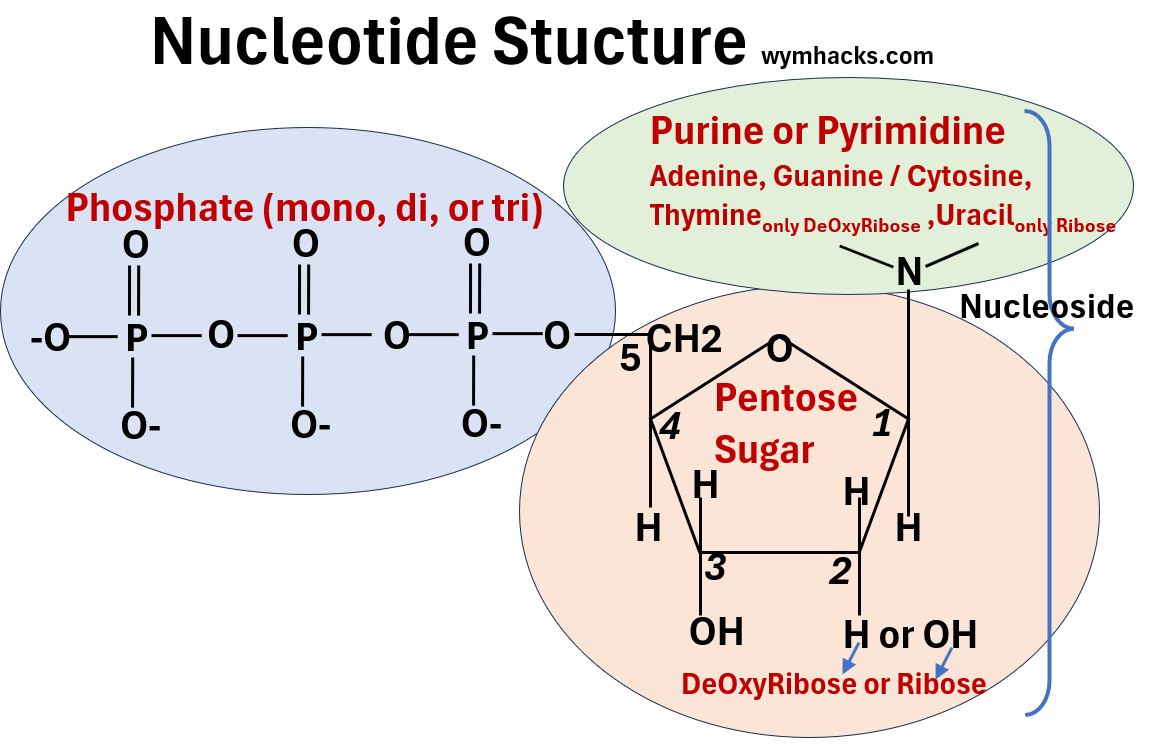
- They are built out of building blocks called Nucleotides.
- The DNA nucleotide shown below is dTMP or DeOxyThymidine Monophosphate. It contains
- a nitrogenous base called Thymine,
- a pentose sugar called DeOxyRibose, and
- an acidic Phosphate group.
Picture_DNA Nucleotide Example_dTMP
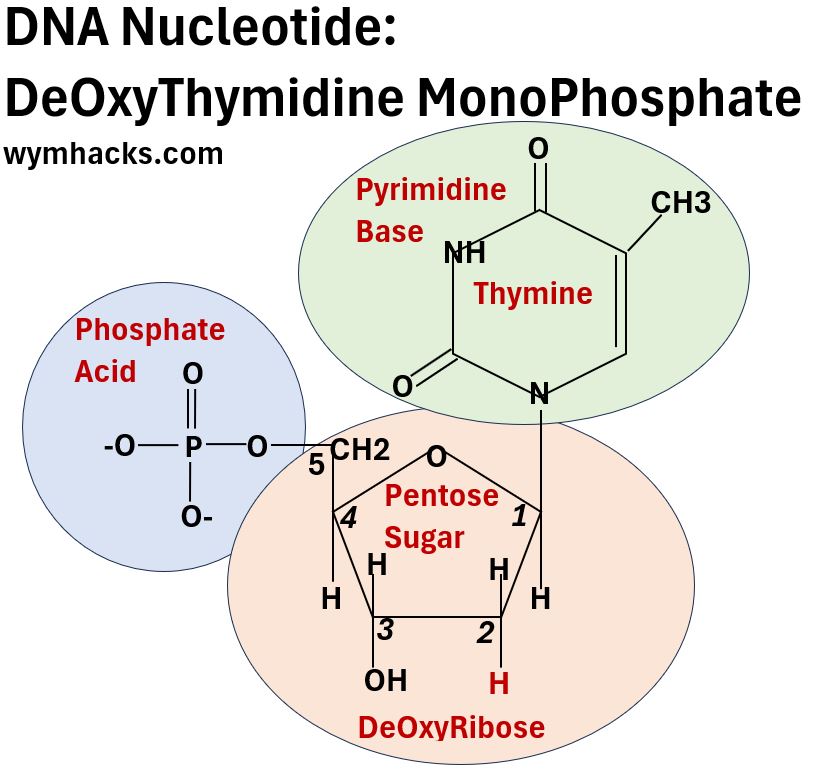
General Structure of Nucleotides
Nucleotides are so instrumental to our biological processes. It’s worth taking a look at their various forms.
See the generic nucleotide pictures version 1 and 2 below.
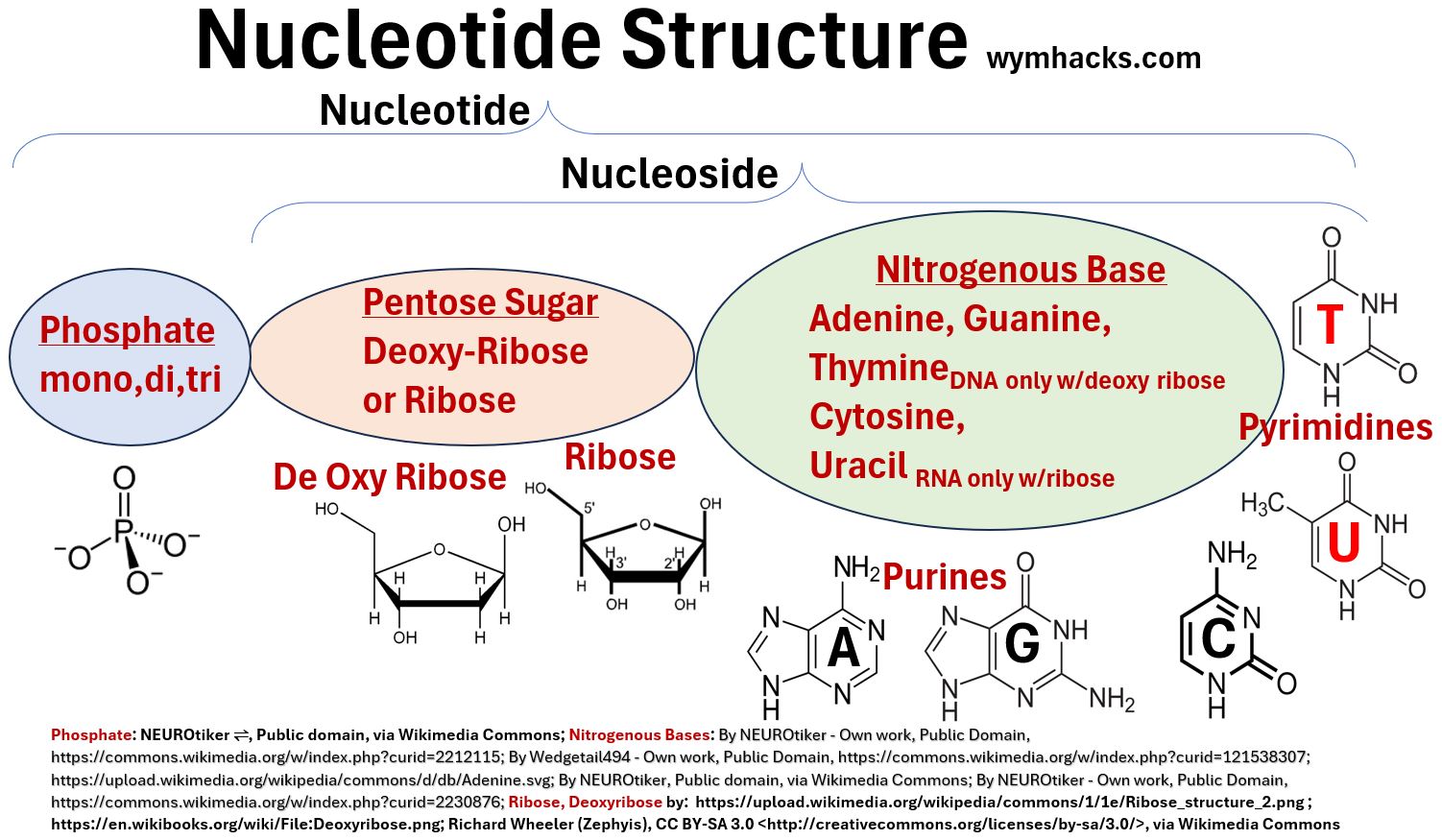
A nucleotide will contain
- 1 of 5 possible nitrogenous base structures
- DNA nucleotides can contain Adenine, Guanine, Cytosine, and Thymine while
- RNA nucleotides can contain Adenine, Guanine, Cytosine, and Uracil.
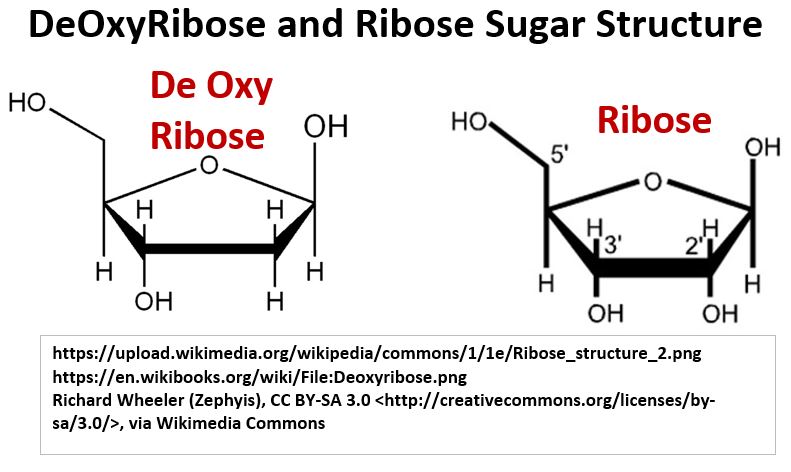
- 1 of 2 pentose sugar structures
- DNA nucleotides contain a Ribose pentose sugar
- RNA nucleotides contain a DeOxyRibose sugar
- 1 of 3 phosphate groups
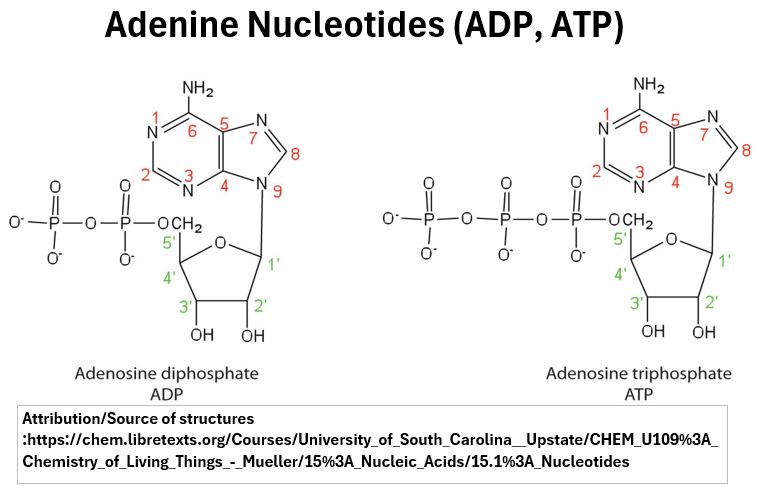
- DNA and RNA will have one phosphate group
- ATP, ADP, and AMP (molecules involved in energy transfer processes in the body) are nucleotides containing three, two, and one phosphate group respectively.
Notice in the pictures below that the Nitrogenous Base + Sugar combinations are called Nucleosides.
Picture_Generic Nucleotide Structure (Version 1)
Picture_Generic Nucleotide Structure (Version 2)
Nucleotide Nitrogenous Bases
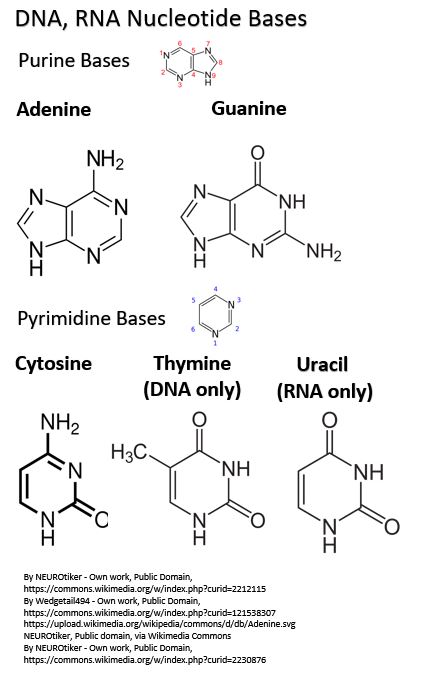
As we noted there are 5 possible nitrogenous bases in a Nucleotide:
- 2 are Purines, found in DNA and RNA
- Adenine – also found in the body’s energy transfer molecules ATP, ADP, AMP, NADH, FADH2, CoEnzyme A
- Guanine – also found in the body’s energy transfer molecule GTP, GDP, GMP
- 3 are Pyrimidines
- Cytosine – found in both RNA and DNA
- Thymine – found in DNA nucleotides but not RNA nucleotides
- Uracil – found in RNA nucleotides but not DNA nucleotides
Picture_Nucleotide Bases
Picture Deoxyribose (in DNA) and Ribose (in RNA)
Nucleotides are named as phosphates of Nucleosides. See the naming convention table below.
Picture_Nucleotide Naming
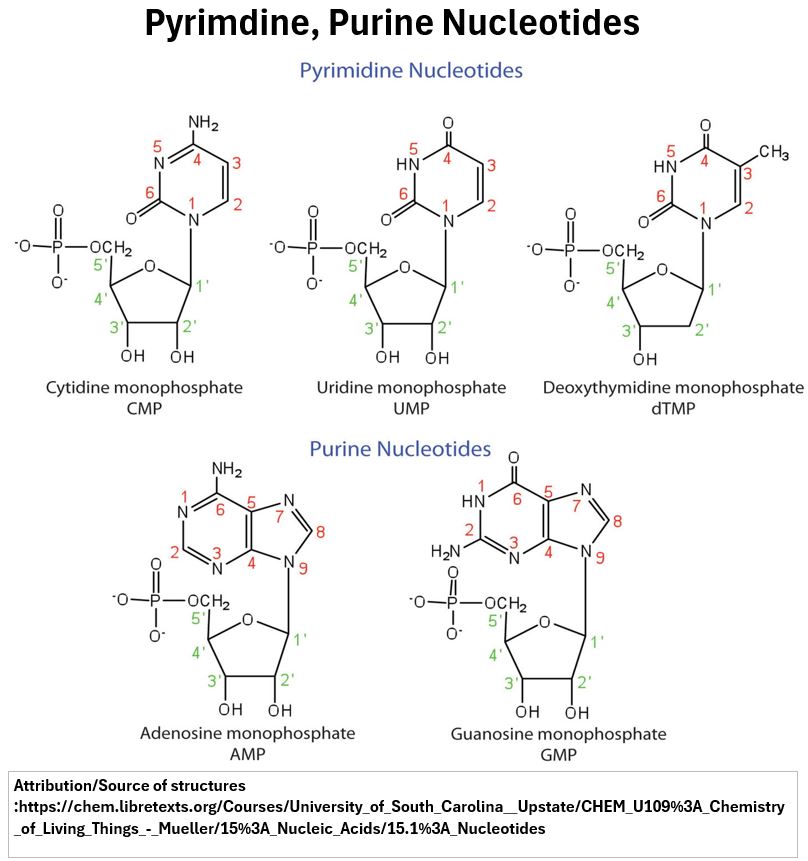
Example Nucleotides
Some examples of nucleotide molecules are shown below.
Picture_Pyrimidine and Purine Nucleotides
Picture_Adenine Nucleotides (ATP, ADP)
DNA and RNA Molecular Structure
- See the pictures of the DNA (DeOxyRiboNucleic Acid) and RNA (RiboNucleic Acid) molecules below.
- DNA molecules look like twisted helixes (helices) connected by rungs (Double Helixes).
- The Rungs consist of pairs of nitrogenous bases (Adenine with Thymine and Guanine with Cytosine)
- while the backbones (the sides of the ladder) consist of alternating pairs of phosphate and deoxyribose groups.
- RNA has a single strand backbone composed of alternating pairs of phosphate and ribose groups.
- The nitrogen bases in RNA are Adenine, Guanine, Cytosine, and Uracil.
- So, we see that RNA has Uracil but not Thymine and vice versa for DNA
Picture_Nucleic Acids – DNA, RNA
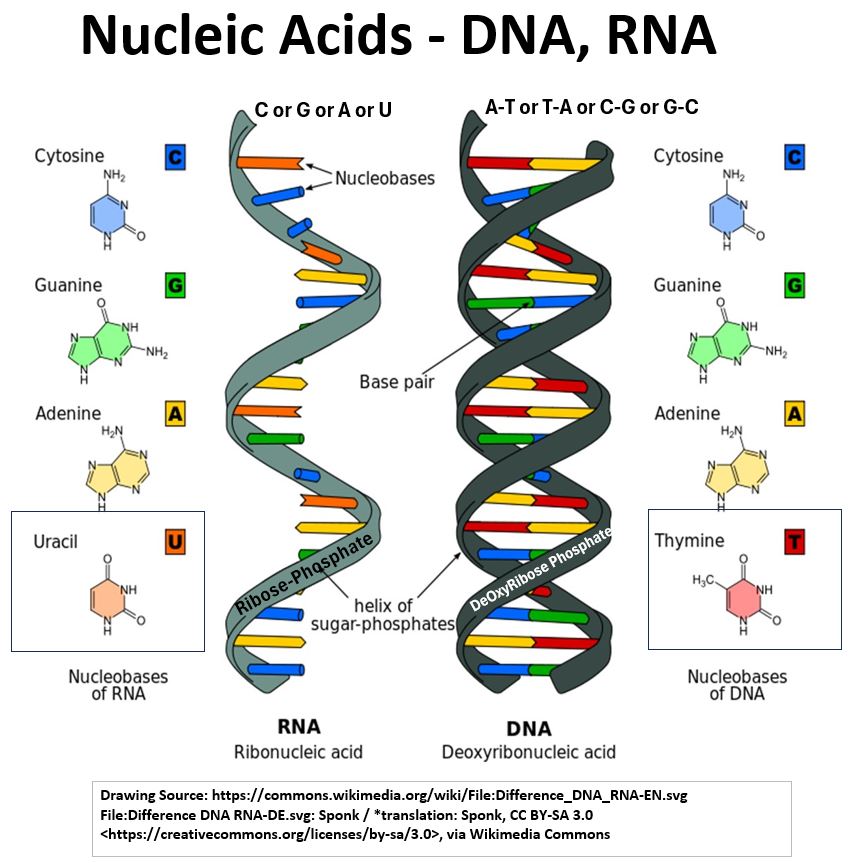
- RNA molecules look like 1/2 of a DNA molecule.
- Like DNA, RNA carries instructions for creating proteins (amino acid sequences).
- Unlike DNA (which mostly resides in the Nucleus of a cell) , RNA is found and can travel throughout the cell.
- RNA travels to Ribosomes in the cytosol of a cell (as Messenger or mRNA).
- There it interacts with Transfer RNA or tRNA to produce proteins.
Vitamins and Minerals
These aren’t typically considered Macro Molecules as they tend to be smaller. (although, as we saw above, Vitamins A, D, E, and K are considered Lipids).
Vitamins and Minerals are critical molecules your body needs but can’t make.
You have to get these through your diet. You can find some good information on dietary guidelines in my articles Diet and Fitness Plan and Nutritional Guidelines with Supplement Comments
Also watch this excellent and brief introduction to Vitamins and Minerals by Sal Khan which I paraphrase below:
- Vitamins are organic
- Examples are Vitamins A, D, E, and K which are fat soluble and Vitamin C which is water soluble
- Minerals are inorganic molecules. Many are critical to the proper functioning of your body (e.g. Phosphorous, Calcium, Potassium, Sodium, Magnesium, Iron, Zinc, etc.)
- Phosphorous is an integral component of our DNA and ATP (a key product of cellular respiration)
- Calcium is integral to our bones and muscle operation (Magnesium is important for muscle operation also).
- Potassium and Sodium are key for neural signaling (transfer of “electro-chemical info” via neurons).
- Iron in hemoglobin binds with Oxygen
- Vitamins (as well as minerals like Magnesium) can also act as co-factors (actors that facilitate the operation of enzymes)
- If you want nice detailed nutritional summaries see the NIH’s Dietary Supplement Fact Sheets.
Disclaimer: The content of this article is intended for general informational and recreational purposes only and is not a substitute for professional “advice”. We are not responsible for your decisions and actions. Refer to our Disclaimer Page.


Hi Waleed,
I really enjoyed your macromolecule summary. Very concise and well researched. It has me interested in the effects of vitamins throughout the body. I know many function as cofactors in enzymatic reactions, but the mechanism of their action would be something fascinating to look into more. Very interesting blog, I look forward to reading more.
Best,
Eric
Hi Eric. Thanks for reading it. I actually have several human biology articles in various stages of completion that I hope to publish soon!
Regards,
Waleed